ABSTRACT
This study describes reported substance use among Kenyan healthcare workers (HCWs), as it has implications for HCWs’ health, productivity, and their ability and likelihood to intervene on substance use. The Alcohol Smoking and Substance Involvement Screening Test (ASSIST) was administered to a convenience sample of HCWs (n = 206) in 15 health facilities. Reported lifetime use was 35.8% for alcohol, 23.5% for tobacco, 9.3% for cannabis, 9.3% for sedatives, 8.8% for cocaine, 6.4% for amphetamine-like stimulants, 5.4% for hallucinogens, 3.4% for inhalants, and 3.9% for opioids. Tobacco and alcohol were also the two most commonly used substances in the previous three months. Male gender and other substance use were key predictors of both lifetime and previous three months’ use rates. HCWs’ substance use rates appear generally higher than those seen in the general population in Kenya, though lower than those reported among many HCWs globally. This pattern of use has implications for both HCWs and their clients.
There has been a recent marked increase in the prevalence of substance use disorders (SUDs) around the world (Whiteford et al. Citation2013). Between 1990 and 2010, there was an increase of 37.6% in the global burden of diseases from mental health and SUDs (Whiteford et al. Citation2013). Using a risk factors approach to the Global Burden of Diseases (GBD), the proportion of the total GBD attributable to specific SUD has increased by 57% for illicit drug use (cannabis, opioids, amphetamines, and injection drug use), 32% for alcohol use, and 3% for tobacco use (Lim et al. Citation2012). Although the GBD for tobacco did not increase much since 1990, in 2010, tobacco use (including second hand smoke) was the second most important risk factor driving the GBD, surpassed only by hypertension (Lim et al. Citation2012). It is projected that, by the year 2020, global tobacco-attributable mortality will be 10% (Mathers and Loncar Citation2006), noteworthy as the highest smoking prevalence is among men in low- and middle-income countries (LMICs) (Alwan Citation2011). Furthermore, alcohol ranked fifth among the risk factors driving the GBD (Lim et al. Citation2012), with about 6% of deaths worldwide directly resulting from alcohol consumption (Mendis et al. Citation2014); in LMICs like Kenya, the availability of cheap, homemade brews propagates unregulated alcohol use and is especially risky as they regularly contain toxic levels of methanol (Lo et al. Citation2013).
Psychoactive substance use exists in all spheres of society (Wu Citation2010), including among healthcare workers (Kenna and Lewis Citation2008). Tobacco and alcohol use among clinicians is of particular concern, as it leads to lower rates of counseling and intervention for these substances (Frank Citation2007; Frank, Breyan, and Elon Citation2000; Oberg and Frank Citation2009). This is important as brief counseling interventions for the management of substance use around the world are effective and of low cost (Barrowclough et al. Citation2001; Dutra et al. Citation2008), and among the few interventions recommended by the WHO as part of the Mental Health Global Action Plan (WHO Citation2010b). Furthermore, HCWs’ substance misuse decreases productivity and increases absenteeism (McFarlin and Fals-Stewart Citation2002), which is of particular concern in LMICs where human resources to provide health services are scarce (WHO et al. Citation2006).
Alcohol and other psychoactive substances are used in both urban and rural areas of Kenya (Atwoli et al. Citation2011; Shaffer et al. Citation2004), with current use among 15- to 65-year-olds of alcohol, tobacco, and cannabis being 13.6%, 9.1%, and 1.0%, respectively (NACADA Citation2012). Current use of the Khat or Miraa plant (which produces an amphetamine-type effect when chewed) is estimated at 4.2% in Kenya (NACADA Citation2012), and is used in other parts of Africa and Europe as well (EMCDDA Citation2011). NACADA data in Kenya indicate that the rate of current use of most substances decreased somewhat between 2007 and 2012 (NACADA Citation2012). However, a recent case study of major urban areas in Kenya points to increasing use of different types of substances, such as Shisha (water pipe smoking) and Kuber (chewing tobacco) (NACADA Citation2014). Another concern is the young age at which substance use is initiated, with many Kenyan youth experimenting with illicit drugs while they are still in primary or secondary school (Kuria Citation1996). This is a phenomenon also seen in the larger sub-Saharan region, and a serious cause for concern (Gore et al. Citation2011).
While there are limited studies providing data on physicians’ or other HCWs’ substance use, both globally and in Kenya, existing data from high-income countries indicate that alcohol and some substance use rates frequently match or exceed those seen in the population (Baldisseri Citation2007; Gastfriend Citation2005). This is contrary to the expectation of some that physicians’ and healthcare workers’ knowledge of the negative health and social consequences of alcohol and substance abuse would reduce use (Kenna and Lewis Citation2008). Alternatively, an understanding of the social context and indication of some likely positive health effects of moderate alcohol use, or the negative stresses arising from high workload and from work-life balance issues, may promote substance use and abuse among healthcare workers (Kenna and Lewis Citation2008; Trinkoff and Storr Citation1998).
Considering the increasing morbidity and mortality caused by alcohol, tobacco, and other substance use disorders worldwide, the impact of HCWs’ personal substance use on their delivery of SUDs-related clinical interventions, and the impact on HCWs’ productivity and absenteeism, it is important to understand the prevalence of SUDs in healthcare workers. It is especially critical in LMICs, particularly in Kenya, where there are currently scant available data. This study sought to describe substance use rates and factors associated with substance use among Kenyan HCWs.
Methods
The study is nested within the Computer-based Drug and Alcohol Training and Assessment in Kenya (eDATA K). eDATA K is a research program of the Africa Mental Health Foundation and NextGenU.org (the only provider of globally free, accredited, higher education courses), and funded by Grand Challenges Canada and the Annenberg Physician Training Program in Addiction Medicine. The goals of eDATA K are to (1) assess the impact of online learning related to alcohol, tobacco, and other substance use disorders on Kenyan primary HCWs, and on the patients attended by these HCWs; and (2) understand the factors that may influence the impact of these online courses. It is this second goal that prompted this study on substance use habits of HCWs.
Ethical approval was granted by the Kenya Medical Research Institute Ethics Review Committee and the University of British Columbia Research Ethics Board. The study was carried out from July to September 2014, with verbal and written consent received prior to data collection. Questionnaires were serialized, for anonymity, before being distributed to respondents who completed and returned them within half a day.
Participants
The study was carried out at 15 facilities: 11 public primary care outpatient clinics in Machakos and Makueni Counties, three private outpatient clinics in Nairobi, and one in Machakos. These facilities were selected for eDATA K based on the following criteria: (1) being a typical facility offering primary care services; (2) staff expressing an interest in the training program; (3) having electricity; and (4) being part of participating eDATA K counties or private healthcare institutions.
All HCWs in the selected facilities’ outpatient services were invited to participate in the eDATA K survey. The minimum sample size requirement to assess the impact of online training on knowledge, skills, and attitudes towards SUDs for HCWs in the larger eDATA K study was 120. A total of 236 HCWs expressed interest in participating in eDATA K, of which 87.3% (206) completed the survey on their substance use. In small, private clinics, public health centers, and dispensaries, at least 80% of staff participated in this study on the prevalence of various substance use; in larger facilities (county and sub-county hospitals outpatients departments), 50–75% of outpatient staff participated.
Measures
In a cross-sectional design, respondents were asked to complete the WHO’s Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST). The ASSIST collects information on, and determines levels of risk from, the use of tobacco products, alcohol, cannabis, amphetamine-type stimulants, cocaine, sedatives and sleeping pills, hallucinogens, opioids, and “other” drugs (Humeniuk et al. Citation2010). The survey takes about five minutes to complete and has been validated for use in LMICs (Humeniuk and Ali Citation2006). Each substance is the object of eight questions to establish its lifetime use (Question 1); frequency of use in the past three months (Question 2); frequency of experiencing a strong desire or urge to use each substance in the last three months (Question 3); frequency of health, social, legal, or financial problems related to substance use in the last three months (Question 4); frequency with which use of each substance has interfered with roles or responsibilities in the past three months (Question 5); whether anyone has ever expressed concern about the respondent’s use of each substance, and how recently that occurred (Question 6); whether the respondent has ever tried to cut down or stop the use of a substance, and failed in that attempt, and how recently that occurred (Question 7); and whether the respondent has ever injected a substance, and how recently that occurred (Question 8).
Responses to questions 2 through 7 of the ASSIST generated a score indicating the level of risk associated with the respondent’s use of each category of substance. Risk was classified as: low risk (0 to 10 for alcohol, and 0 to 3 for all other substances); moderate risk (11 to 26 for alcohol, and 4 to 26 for the other substances); and high risk (27 and above) (Humeniuk et al. Citation2010).
To adapt the survey to Kenya and to enable the respondents to properly understand the various categories of substances covered by the ASSIST, examples of substances and their local or colloquial names were included; e.g., amphetamine-type stimulants (miraa/mairungi, khat, kangeta, muguka, katepa, kirembe, mafuta, giza, majani, veve, uppers, pep pills, bennies, speed, ecstasy). A survey of socio-demographic information was also included. The questionnaires were administered in English.
Data management and statistical analysis
All data were double-entered and assessed for quality and outliers. Data analyses were undertaken using IBM SPSS® Version 21. The first analytic stage established the basic descriptive statistics (means and standard deviations for numerical variables and frequencies for nominal and ordinal variables). For tobacco, alcohol, cannabis, and cocaine, the four most prevalent substances used in the last three months, logistic regression models were used to assess the association and odds ratios between the use of a given substance and the use of the other substances, as well as associations with the various available socio-demographic factors. To minimize the exclusion of cases from missing answers to survey questions, we performed regression analyses on the original and a multiple imputation data set, and the regression analysis presents the results of the fifth and last iteration.
Dependent variables were lifetime use and previous three months’ use. Lifetime use was defined as an affirmative response to Question 1 of the ASSIST: “In your life which of the following substances have you ever used (non-medical use only)?” Previous three months’ use was derived from the following question: “In the past 3 months, how often have you used the substances you mentioned?” with responses coded to include all respondents who used a given substance at least once in that time period. Independent variables were age, a socioeconomic status (SES) index based on ownership of assets (mobile phone, bicycle, motorbike, car), facility type (private outpatient clinic, public health center, public hospital outpatient clinic), region (Nairobi, Machakos, Makueni), occupation (clinician, non-clinician), gender (male, female), education (secondary school, certificate, diploma, degree), and marital status (married and non-married—a combination of single, cohabiting, divorced/separated, or widow/widower).
Multivariate models included variables significantly associated with consumption at the bivariate level for at least one of the substance use of interest, as well as demographic variables typically associated with substance use (age, gender, education, and SES), and absent correlations >0.4 (an indicator of collinearity) between included variables (from Spearman’s rank-order correlation co-efficient (rs)) (Dawson and Trapp Citation2004; Tu et al. Citation2005). Unadjusted odds ratios (OR) and adjusted odds ratios (AOR) with 95% confidence intervals were estimated from the logistic regression models. The level of statistical significance was set at p ≤ 0.05, and all tests were two-sided.
Results
Demographic characteristics
Of the 206 respondents, nurses, clinical officers (those with a Diploma in Medicine, enabling them to practice at a level similar to nurse practitioners or physician assistants in other countries), and medical doctors were categorized as clinicians (45.1%), with all other cadres of staff categorized as non-clinicians (54.9%), including community health workers, laboratory technicians, receptionists, and other support staff. Clinicians were educated to certificate (32.6%), diploma (35.9%), and undergraduate degree (31.5%) levels. Slightly more than half of non-clinicians held certificates (55.6%), 4.6% held diplomas, 10.2% had undergraduate degrees, and 29.6% had only a secondary school education. Three-quarters (75.7%) of respondents were working in public (government-owned) facilities; the remainder worked in private facilities. The majority (63.1%) of respondents were female. The mean age was 35.3 years (SD = 10.1), with a range of 20 to 58 years.
Almost all (97.6%) respondents indicated that they were Christian, with the rest reporting that they were Buddhist (1.5%) or Muslim (1.0%). Most respondents (87.6%) worked full-time and, of those who worked part-time, 73% said they also ran a business on the side. Almost all respondents (95.9%) reported owning a mobile phone, while only 9.8% owned a motor vehicle. Two-thirds (67.3%) indicated that they were married, and most of the remainder were single (29.3%).
Healthcare workers’ substance use rates
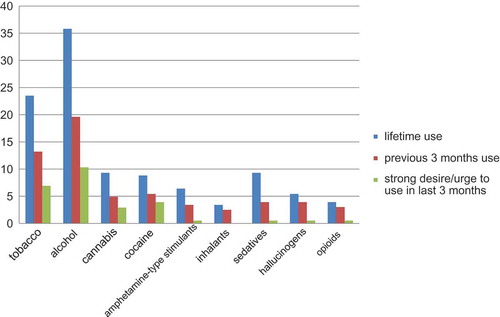
The lifetime substance use rate was 35.8% for alcohol, 23.5% for tobacco, 9.3% for cannabis and sedatives, 8.8% for cocaine, 6.4% for amphetamine-like stimulants, 5.4% for hallucinogens, 3.9% for opioids, and 3.4% for inhalants. Alcohol was the substance most frequently used in the previous three months (19.6%), with tobacco at 13.2%, cocaine at 5.4%, cannabis at 4.9%, sedatives and hallucinogens at 3.9%, amphetamine-type stimulants at 3.4%, and opioids at 3.0%. Respondents who used in the last three months were asked how often they had a strong desire or urge to use various substances in the previous three months. More than half of those who used alcohol, tobacco, cannabis, or cocaine in the last three months reported they had a strong desire or urge to use during that period. shows the distribution of substance use rates. shows the breakdown of substance use frequency by gender.
Table 1. Substance use rates, by gender, among Kenyan healthcare workers.
Lifetime use for men was higher than that of women for all substances except hallucinogens. Previous three months’ use followed a similar trend with usage rates higher for all substances among men, with the exception of previous three months’ use of sedatives, which was higher among women.
Analysis of ASSIST scores revealed that most HCWs had a low level of risk from their substance use. Tobacco was the only substance with a HCW reaching a high risk score (0.5%). Moderate risk use was observed at 11.8% for tobacco, 4.4% for cocaine, 3.4% for cannabis and sedatives, 2.9% for alcohol and hallucinogens, 2.5% for amphetamine-type stimulants, and 1.5% for opioids. Tobacco and alcohol were the substances for which the highest proportion of workers reported they were experiencing negative health, social, financial, or legal consequences from their use in the last three months (3.9 and 2.0%, respectively, for tobacco and alcohol); 4.4% and 2%, respectively, received expressions of concern; 6.4% and 3.5%, respectively, tried to cut down without succeeding; and 1.0% of failed to fulfill their roles due to their alcohol use (ASSIST 4 to 7). For the other substances, negative consequences had been experienced by 1 to 2.5% of workers, none had failed to fulfill their roles, 0.5% received expression of concern for cannabis or sedatives use, and only 1% of workers tried to cut cannabis use without success, with none for all of the other substances. Only 1% of HCWs reported having ever used any recreational drug by injection, which included injecting amphetamine-type substances, sedatives, and/or hallucinogens.
Regression analysis
We performed logistic regression to examine the association between hypothesized risk and protective factors for lifetime use and previous three months’ use for four common substances (tobacco, alcohol, cannabis, and cocaine). presents the bivariate odds ratios, while presents the multivariate analysis (Adjusted Odds Ratios, AOR).
Table 2. Unadjusted odds ratios of Kenyan health workers’ characteristics in relation to their lifetime and previous three months’ substance use.
Table 3. Adjusted odds ratio of variables associated with lifetime and previous three months’ substance use among healthcare workers in Kenya.
For tobacco use in the lifetime multivariate model, only four variables remained significant, with an AOR respectively of 17.82 (CI 6.10–47.13, p < 0.001) for lifetime use of alcohol, 4.87 (CI:1.31–18.06, p = 0.018) for lifetime cannabis use, 2.61 (CI:1.01–6.72, p = 0.048) for being male, and 1.28 (1.04–1.56, p = 0.02) for the SES Index. Lifetime cocaine use was associated with lifetime tobacco use only in the bivariate model. For the last three months’ model, the previous three months’ use of alcohol (AOR:7.58; CI:2.48–23.20, p < 0.000) and cannabis (AOR:8.71; CI:1.04–73.00, p = 0.018), as well as male gender (AOR:4.87; CI:1.52–15.66, p = 0.048), remained statistically significant in both bivariate and multivariate analysis, while the SES index reached statistical significance only in bivariate analysis.
For alcohol use, in the lifetime multivariate model, significant AOR included lifetime tobacco use, 16.95 (CI:6.10–36.37, p < 0.000), and male gender, 2.54 (CI:1.16–5.28, p = 0.02), while the increased OR in those ≥50 vs. 20–29 years old was significant only in bivariate analysis. The model for the last three months’ use of alcohol included a significant AOR for those who used tobacco in the last three months, 7.68 (CI:2.43–24.29, p = 0.001), and male gender, 3.91 (CI:1.57–9.74, p = 0.003), while cannabis and cocaine use in the last three months were only significant in bivariate analysis.
For lifetime cannabis use, lifetime use of tobacco, gender, and SES index were statistically significant in multivariate analysis; while in bivariate analysis lifetime alcohol and cocaine use were also significant, and the SES index was not. For the past three months’ cannabis use, only tobacco and alcohol use were statistically significant in both the multivariate and the bivariate analysis.
For the lifetime and past three months’ cocaine use models, none of the significant variables in bivariate analysis remained significant in the multivariate analysis.
Discussion
This study fills an important literature gap regarding the prevalence of substance use among HCWs in Kenya and could be indicative of HCW substance use in other similar LMICs. This study is particularly important since most studies of healthcare workers’ alcohol, tobacco, and other substance use has been conducted in a few high-income countries. A strength of our study is the very high response rate, meaning the inclusion of the vast majority of health workers in each clinic. In that sample, we found lifetime and current use rates of most substances lower than that of the general population in most HICs (WHO Citation2010a). However, these rates were much higher than that reported by the Kenyan population: lifetime use 1.6 times higher for tobacco in males and 1.9 times higher for females; for alcohol 1.4 times higher in males and 5.9 times higher in females; cocaine 23.6 times higher in males and 10 times higher in females; cannabis 2.2 times higher in males and 1.1 times higher in females; and inhalants 10.6 times higher in males and 2.3 times higher in females (NACADA Citation2012). While the NACADA study did not look at hallucinogens, sedatives, or opioids in the same way as did the ASSIST, the NACADA-reported rates of other substance use (which should include hallucinogens), prescription drug abuse (which should include sedative and prescription opiates), and heroin use are much lower in the general population than in our study of HCWs.
Use of alcohol, tobacco, and these other substances, even at low to moderate risk levels, may exert a higher total burden on the public health system than harmful, high-risk use (Humeniuk et al. Citation2010), due to the large proportion of the population at stake falling in the low to moderate risk categories. This is especially concerning, as the use of tobacco, alcohol, and many other substances, even at these levels, remains associated with health problems of measurable burden at population levels, compared to the people categorized as high-risk users who might individually experience higher disease burden, but not contribute as much to the global burden due to their smaller number. As an example, long-term low to moderate alcohol use is associated with liver disease and pancreatitis (Warren and Murray Citation2013), while short-term low-risk alcohol use is associated with risky sexual behavior (Thompson et al. Citation2014) and motor-vehicle injuries (Thomas and Rockwood Citation2001). Acute cannabis use can affect cognition for weeks after its use, and long-term (even occasional) cannabis use is associated with long-term cognitive impairment (Crean et al. Citation2011).
Additionally, healthcare workers’ lifestyle choices affect their patients’ health practices (Frank et al. Citation2013; Oberg and Frank Citation2009), including their reported substance use (Frank et al. Citation2008; Voltmer, Frank, and Spahn Citation2013). This means that having optimal substance use behaviors among HCWs can optimize outcomes for whole patient populations.
Reported substance use rates were higher among male HCWs than female HCWs for all substances—with the exception of hallucinogens for lifetime use and sedatives for past three months’ use. This is similar to the findings from NACADA (Citation2012) and other studies conducted on the Kenyan general population (Atwoli et al. Citation2011; Kinoti, Jason, and Harper Citation2011; Odek-Ogunde and Pande-Leak Citation1999; Othieno, Kathuku, and Ndetei Citation2000), as well as specific studies of HCWs from other countries (Frank, Elon, and Hertzberg Citation2007; Frank and Segura Citation2009; Underwood and Fox Citation2000). This might be explained by lower peer pressure to use (Borsari and Carey Citation2006), greater social sanctions for substance use or abuse (Nolen-Hoeksema Citation2004), and increased susceptibility to negative effects of some substance use in females compared to males (Nolen-Hoeksema Citation2004). While female HCWs in our study report low levels of substance use compared to that of females in HICs, they report higher rates of substance use than other Kenyan women and women from many LMICs; and higher rates than Kenyan male HCWs for sedatives (WHO Citation2010a; NACADA Citation2012). The reasons for this are unknown and warrant further study.
Socio-economic indicators (SES index, education, occupation) were associated with substance use in bivariate analysis in this study. This is similar to the NACADA findings showing that 19.8% of people in the highest income group in Kenya currently use alcohol compared with 13.2% in the lowest income group, a frequently observed association, especially for those with a college education (Atwoli et al. Citation2011; NACADA Citation2012; Odek-Ogunde and Pande-Leak Citation1999). However, a previous study in Kenya showed that lower levels of education and poor employment status were risk factors for cannabis use (Kinoti, Jason, and Harper Citation2011). Neither region nor age were significantly associated with substance use, contrasting with other Kenyan and global studies showing that increasing age is a risk factor for alcohol consumption in the general population (Lo et al. Citation2013). Studies also show higher use rates with increasing age among medical students and physicians (Flaherty and Richman Citation1993; Frank et al. Citation2008).
As in other studies of co-occurring substance use in the general population (Akre et al. Citation2010; Lee, Martin, and Kelly Citation2014; McKetin et al. Citation2014; Rodríguez-Álvarez et al. Citation2015), our study showed that consumption of alcohol, tobacco, cannabis, or cocaine significantly increased the odds of at least one of the other substances being used.
Even though this study used a relatively small convenience sample, a large proportion of HCWs in the targeted departments of the participating health facilities responded to the survey. Furthermore, despite the potential for social desirability bias, many HCWs reported the use of substances identified as stigmatized (Room Citation2005). The report of use of stigmatized substances in relatively high proportion further supports this study as providing a relatively valid representation of substance use among Kenyan HCWs of these regions.
These findings indicate the need for a more rigorous assessment of substance use, abuse, and dependence in HCWs in Kenya and other LMICs, since there are few existing studies, and because HCWs’ personal substance use affects their provision of substance-use-related care (Frank Citation2007; Frank, Breyan, and Elon Citation2000; Oberg and Frank Citation2009). Furthermore, it cannot be assumed that HCWs understand the consequences of their own consumption, as there is limited substance use training available, and there had been no training on the topic for our particular sample of community health workers (Hitchen, Tairyan, and Clair Citation2014). These findings indicate the need for training and intervention for HCWs who abuse substances, a need rarely addressed in many LMICs.
Funding
The authors acknowledge funding from Grand Challenges Canada–GCC (GMH 0092-04), Canada Research Chair Program, Annenberg Physician Training Program in Addiction Medicine.
Additional information
Funding
References
- Akre, C., P. Michaud, A. Berchtold, and J. Suris. 2010. Cannabis and tobacco use: Where are the boundaries? A qualitative study on cannabis consumption modes among adolescents. Health Education Research 25 (1):74–82. doi:10.1093/her/cyp027.
- Alwan, A. 2011. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: World Health Organization.
- Atwoli, L., P. Mungla, M. Ndung’u, K. Kinoti, and E. Ogot. 2011. Prevalence of substance use among college students in Eldoret, Western Kenya. BMC Psychiatry 11 (1):34. doi:10.1186/1471-244X-11-34.
- Baldisseri, M. 2007. Impaired healthcare professional. Critical Care Medicine 35 (2 Suppl):S106–16. doi:10.1097/01.CCM.0000252918.87746.96.
- Barrowclough, C., G. Haddock, N. Tarrier, S. Lewis, J. Moring, R. O’Brien, N. Schofield, and J. McGovern. 2001. Randomized controlled trial of motivational interviewing, cognitive behavior therapy, and family intervention for patients with comorbid schizophrenia and substance use disorders. American Journal of Psychiatry 158 (10):1706–13. doi:10.1176/appi.ajp.158.10.1706.
- Borsari, B., and K. Carey. 2006. How the quality of peer relationships influences college alcohol use. Drug and Alcohol Review 25 (4):361–70. doi:10.1080/09595230600741339.
- Crean, R., N. Crane, and B. Mason. 2011. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. Journal of Addiction Medicine 5 (1):1–8. doi:10.1097/ADM.0b013e31820c23fa.
- Dawson, B., and R. Trapp. 2004. Basic & clinical biostatistics, 4th ed. Columbus, OH: McGraw Hill Professional.
- Dutra, L., G. Stathopoulou, S. Basden, T. Leyro, M. Powers, and M. Otto. 2008. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry 165 (2):179–87. doi:10.1176/appi.ajp.2007.06111851.
- EMCDDA (European Monitoring Centre for Drugs and Drug Addiction). 2011. 2011 annual report. European Monitoring Centre for Drugs and Drug Addiction. http://www.emcdda.europa.eu/online/annual-report/2011/cannabis/3.
- Flaherty, J., and J. Richman. 1993. Substance use and addiction among medical students, residents, and physicians. Psychiatric Clinics of North America 16 (1):189–97.
- Frank, E. 2007. We physicians preach what we practice, and that matters. Medscape General Medicine 9 (4):59.
- Frank, E., J. Breyan, and L. Elon. 2000. Physician disclosure of healthy personal behaviors improves credibility and ability to motivate. Archives of Family Medicine 9 (3):287–90. doi:10.1001/archfami.9.3.287.
- Frank, E., Y. Dresner, M. Shani, and S. Vinker. 2013. The association between physicians’ and patients’ preventive health practices. CMAJ: Canadian Medical Association Journal 185 (8):649–53. doi:10.1503/cmaj.121028.
- Frank, E., L. Elon, and V. Hertzberg. 2007. A quantitative assessment of a four-year intervention that improved patient counseling through improving medical student health. Medscape General Medicine 9 (2):58.
- Frank, E., L. Elon, T. Naimi, and R. Brewer. 2008. Alcohol consumption and alcohol counselling behaviour among US medical students: Cohort study. BMJ 337 (1):a2155. doi:10.1136/bmj.a2155.
- Frank, E., and C. Segura. 2009. Health practices of Canadian physicians. Canadian Family Physician 55 (8):810–11.e7.
- Gastfriend, D. 2005. Physician substance abuse and recovery: What does it mean for physicians—And everyone else? JAMA 293 (12):1513–15. doi:10.1001/jama.293.12.1513.
- Gore, F., P. Bloem, G. Patton, J. Ferguson, V. Joseph, C. Coffey, S. Sawyer, and C. Mathers. 2011. Global burden of disease in young people aged 10–24 years: A systematic analysis. The Lancet 377 (9783):2093–102. doi:10.1016/S0140-6736(11)60512-6.
- Hitchen, C., K. Tairyan, and V. Clair 2014. Piloting computer based training on substance use disorders for primary care in Kenya: A developmental evaluation of eData K. Capstone project report, Burnaby, Canada: Simon Fraser University.
- Humeniuk, R., and R. Ali. 2006. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) and pilot brief intervention: A technical report of phase II findings of the WHO ASSIST Project. Geneva, Switzerland: World Health Organization. http://www.who.int/substance_abuse/activities/assist_technicalreport_phase2_final.pdf
- Humeniuk, R., R. Ali, V. Poznyak, and M. Monteiro. 2010. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): Manual for use in primary care. Geneva, Switzerland: World Health Organization.
- Kenna, G., and D. Lewis. 2008. Risk factors for alcohol and other drug use by healthcare professionals. Substance Abuse Treatment, Prevention, and Policy 3 (1):3. doi:10.1186/1747-597X-3-3.
- Kinoti, K., L. Jason, and G. Harper. 2011. Determinants of alcohol, khat, and bhang use in rural Kenya. African Journal of Drug and Alcohol Studies 10 (2):107–18.
- Kuria, M. 1996. Drug abuse among urban as compared to rural secondary schools students in Kenya: A short communication. East African Medical Journal 73 (5):339.
- Lee, D., C. Martin, and T. Kelly. 2014. Acute effect of alcohol on inhibitory control and subsequent tobacco use in young adult occasional smokers. Drug & Alcohol Dependence 140 (July):e118. doi:10.1016/j.drugalcdep.2014.02.337.
- Lim, S., T. Vos, A. Flaxman, G. Danaei, K. Shibuya, H. Adair-Rohani, M. AlMazroa, et al. 2012. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet 380 (9859):2224–60. doi:10.1016/S0140-6736(12)61766-8.
- Lo, T., J. Oeltmann, F. Odhiambo, C. Beynon, E. Pevzner, K. Cain, K. Laserson, and P. Phillips-Howard. 2013. Alcohol use, drunkenness and tobacco smoking in rural Western Kenya. Tropical Medicine & International Health: TM & IH 18 (4):506–15. doi:10.1111/tmi.12066.
- Mathers, C., and D. Loncar. 2006. Projections of global mortality and burden of disease from 2002 to 2030. Plos Medicine 3 (11):e442. doi:10.1371/journal.pmed.0030442.
- McFarlin, S., and W. Fals-Stewart. 2002. Workplace absenteeism and alcohol use: A sequential analysis. Psychology of Addictive Behaviors 16 (1):17–21. doi:10.1037/0893-164X.16.1.17.
- McKetin, R., J. Chalmers, M. Sunderland, and D. Bright. 2014. Recreational drug use and binge drinking: Stimulant but not cannabis intoxication is associated with excessive alcohol consumption. Drug and Alcohol Review 33 (4):436–45. doi:10.1111/dar.12147.
- Mendis, S., T. Armstrong, D. Beticher, F. Branca, J. Lauer, C. Mace, V. Poznyak, L. Riley, V. Da Costa Silva, and G. Stevens. 2014. Global status report on noncommunicable diseases 2014. Geneva, Switzerland: World Health Organization.
- NACADA. 2012. Rapid assessment of drugs and substance abuse in Kenya. http://www.nacada.go.ke/documents-and-resources/category/8-research-survey-findings?download=40:rapid-assessment-of-alcohol-and-drug-abuse-situation-in-kenya-2012
- NACADA. 2014. Trends and patterns of emerging drugs in Kenya: A case study of Mombasa and Nairobi counties. NAC/10/2014. Nairobi, Kenya: National Authority for the Campaign against Alcohol and Drug Abuse.
- Nolen-Hoeksema, S. 2004. Gender differences in risk factors and consequences for alcohol use and problems. Clinical Psychology Review 24 (8):981–1010. doi:10.1016/j.cpr.2004.08.003.
- Oberg, E., and E. Frank. 2009. Physicians’ health practices strongly influence patient health practices. The Journal of the Royal College of Physicians of Edinburgh 39 (4):290–91. doi:10.4997/JRCPE.2009.422.
- Odek-Ogunde, M., and D. Pande-Leak. 1999. Prevalence of substance use among students in a Kenyan university: A preliminary report. East African Medical Journal 76 (6):301–06.
- Othieno, C., D. Kathuku, and D. Ndetei. 2000. Substance abuse in outpatients attending rural and urban health centres in Kenya. East African Medical Journal 77 (11):592–95.
- Rodríguez-Álvarez, T., I. Racamonde, I. González-Mariño, A. Borsotti, R. Rodil, I. Rodríguez, E. Zuccato, J. Quintana, and S. Castiglioni. 2015. Alcohol and cocaine co-consumption in two European cities assessed by wastewater analysis. Science of the Total Environment 536 (December):91–98. doi:10.1016/j.scitotenv.2015.07.016.
- Room, R. 2005. Stigma, social inequality and alcohol and drug use. Drug and Alcohol Review 24 (2):143–55. doi:10.1080/09595230500102434.
- Shaffer, D., R. Njeri, A. Justice, W. Odero, and W. Tierney. 2004. Alcohol abuse among patients with and without HIV infection attending public clinics in Western Kenya. East African Medical Journal 81 (11):594–98.
- Thomas, V., and K. Rockwood. 2001. Alcohol abuse, cognitive impairment, and mortality among older people. Journal of the American Geriatrics Society 49 (4):415–20. doi:10.1046/j.1532-5415.2001.49085.x.
- Thompson, R., N. Eaton, M. Hu, B. Grant, and D. Hasin. 2014. Regularly drinking alcohol before sex in the United States: Effects of relationship status and alcohol use disorders. Drug and Alcohol Dependence 141:167–70. doi:10.1016/j.drugalcdep.2014.05.021.
- Trinkoff, A., and C. Storr. 1998. Work schedule characteristics and substance use in nurses. American Journal of Industrial Medicine 34 (3):266–71. doi:10.1002/(ISSN)1097-0274.
- Tu, Y., M. Kellett, V. Clerehugh, and M. Gilthorpe. 2005. Problems of correlations between explanatory variables in multiple regression analyses in the dental literature. British Dental Journal 199 (7):457–61. doi:10.1038/sj.bdj.4812743.
- Underwood, B., and K. Fox. 2000. Law and ethics: A survey of alcohol and drug use among UK-based dental undergraduates. British Dental Journal 189 (6):314–17. doi:10.1038/sj.bdj.4800756.
- Voltmer, E., E. Frank, and C. Spahn. 2013. Personal health practices and patient counseling of German physicians in private practice. International Scholarly Research Notices 2013 (August):e176020. doi:10.5402/2013/176020.
- Warren, K., and M. Murray. 2013. Alcoholic liver disease and pancreatitis: Global health problems being addressed by the U.S. National Institute on Alcohol Abuse and Alcoholism. Journal of Gastroenterology and Hepatology 28 (S1):4–6. doi:10.1111/jgh.2013.28.issue-s1.
- Whiteford, H., L. Degenhardt, J. Rehm, A. Baxter, A. Ferrari, H. Erskine, F. Charlson, et al. 2013. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. The Lancet 382 (9904):1575–86. doi:10.1016/S0140-6736(13)61611-6.
- WHO. 2010a. Atlas on substance use (2010): Resources for the prevention and treatment of substance use disorders. Geneva, Switzerland: World Health Organization.
- WHO. 2010b. mhGAP intervention guide for mental, neurological and substance use disorders in non-specialized health settings. Geneva, Switzerland: World Health Organization.
- WHO et al. 2006. The World Health Report: 2006: Working together for health. http://apps.who.int/iris/handle/10665/43432
- Wu, L.-T. 2010. Substance abuse and rehabilitation: Responding to the global burden of diseases attributable to substance abuse. Substance Abuse and Rehabilitation 2010 (1):5–11. doi:10.2147/SAR.S14898.