 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Despite various interventions available for substance use disorders, relapse rates remain substantial and, therefore, alternative strategies for attenuating dependence are needed. This study examined the associations between exercise frequency, illicit substance use, and dependence severity among a large sample of people who use drugs. The study utilized data from the Global Drug Survey 2018 (N = 57,110) to investigate the relationship between exercise frequency, illicit substance use, and substance dependence severity. Binomial regressions were employed to examine the relationship between exercise and SDS scores for 9 drugs. Greater exercise frequency correlated with reduced severity of substance dependence for specific drugs: cannabis (χ2 = 14.75, p < .001), MDMA (χ2 = 4.73, p = .029), cocaine (χ2 = 8.37, p = .015), amphetamine powder (χ2 = 6.39, p = .041), and methamphetamine (χ2 = 15.17, p < .001). These findings suggest a potential link between exercise and reduced substance use dependency. Further research is needed to understand the complex dynamics between exercise and substance use, considering potential bidirectional relationships and concurrent factors.
Introduction
Substance use disorders (SUDs) pose a substantial public health burden (Castelpietra et al. Citation2022), with high relapse rates post-treatment (Brandon, Vidrine, and Litvin Citation2007). Emerging as a supplementary approach for SUD treatment, exercise garners interest due to its potential to prevent relapse (Marlatt and Donovan Citation2005). Despite lower adherence to physical activity guidelines among illicit substance users (Linke and Ussher Citation2015; Smith and Lynch Citation2012), there is a growing inclination toward regular exercise (Furzer et al. Citation2021; Ramadas et al. Citation2021). Various facets of physical fitness, including cardiorespiratory fitness and muscular strength, influence overall health (Garber et al. Citation2011; Teixeira et al. Citation2012) and may mitigate mental and physical health issues (Sujkowski et al. Citation2022). The theoretical grounding for this relationship posits that exercise impacts similar brain receptors as psychoactive drugs, potentially leading to a reduction in the severity of dependence on substances by providing an alternative means of influencing those receptors (Lynch et al. Citation2013).
While there are different types of SUDs corresponding to specific substances, such as alcohol, cannabis, opioids, and others, there are shared underlying mechanisms in addiction that cut across all substances (Volkow et al. Citation2009). Sherman (Citation2017) suggests that a typical process in the brain involves the release, reuptake and reentry of neurotransmitters, which helps maintain their levels in the synaptic space. Similarly, a substantial body of evidence has shown that exercise has an impact on brain receptors that are also affected by psychoactive drugs (Basso and Suzuki Citation2017; Heijnen et al. Citation2016; Lynch et al. Citation2013; Saanijoki et al. Citation2018). Research has demonstrated that physical exercise triggers the release of endogenous opioids (endorphins) in the frontolimbic brain regions, which is associated with the perceived euphoria often experienced by runners (Boecker et al. Citation2008; Desai et al. Citation2022). Exercise such as cycling (Sparling et al., Citation2003) and running (Raichlen et al. Citation2012) significantly increase the release of endocannabinoids. As a result of these interactions, other studies have suggested that exercise may prevent dependency formation (Buchowski et al. Citation2011; Roessler Citation2010; Vidot et al. Citation2019; Zhu et al. Citation2018). Specifically, engaging in regular exercise may act as a protective factor against the development of substance use dependency and, consequently, a lack of physical activity might elevate the vulnerability to engage in illicit substance use, potentially contributing to an increased risk of disorders (Buchowski et al. Citation2011; Roessler Citation2010; Vidot et al. Citation2019; Zhu et al. Citation2018).
Exercise, which falls under the umbrella of physical activity, comprises structured actions aimed at improving or sustaining physical fitness (Wasfy and Baggish Citation2016). It is often grouped into aerobic/endurance (e.g., running, walking) and resistance (e.g., weight lifting) activities, although various sports combine these physiological aspects (Wasfy and Baggish Citation2016). Each facet of physical fitness, encompassing elements such as cardiorespiratory fitness, muscular strength and endurance (muscular fitness), body composition, flexibility, and neuromotor fitness, has the potential to impact various facets of health (Garber et al. Citation2011; Teixeira et al. Citation2012). As a result, exercise offers a range of benefits to both the mind and body, serving as a protective factor against illnesses (Sujkowski et al. Citation2022). This pattern of protection often extends to other health behaviors, with individuals who engage in exercise also tending to adopt habits and abstaining from substance use (Leasure et al. Citation2015). Most recently, engaging in enjoyable and structured exercise has been associated with improved health indicators and reduced likelihood of relapse among individuals undergoing SUD treatment (Furzer et al. Citation2021). Drawing these findings together, we underscore the potential significance of incorporating enjoyable exercise as a crucial component in effectively meeting illicit substance use dependency. Engaging in exercise can serve as a protective factor against craving and relapse by exposing individuals to novel environments, thus reducing exposure to cues associated with substance use (Ehrman et al. Citation1992; Ramadas et al. Citation2021). Participation in meaningful and structured activities, including exercise, is a crucial element in overcoming SUDs, as it offers opportunities for establishing new routines, fostering social connections, and facilitating identity transformation (Piatkowski et al. Citation2020; Ramadas et al. Citation2021). Notably, research findings indicate that individuals who undertake exercise (possibly as a coping mechanism) are less inclined to resort to alcohol consumption for dealing with negative emotions (Weinstock et al. Citation2017).
Therefore, this study examined the associations among exercise frequency, illicit substance use, and severity of dependence in a sample of people who use drugs from the Global Drug Survey (GDS). We hypothesized, firstly, that individuals who report engaging in a higher frequency of exercise would attain lower scores on the Severity of Dependence Scale (SDS) for nine illicit substances (cannabis, MDMA, cocaine, amphetamine (powder and paste), methamphetamine, ketamine, GHB and mephedrone). Secondly, we proposed that this relationship would remain significant for those participants who exclusively used a single illicit substance in the last 12 months out of the nine substances for which SDS scores were obtained. This proposition is rooted in the idea that the distinct effects of exercise on substance use dependency might be more pronounced and discernible among those who have engaged in a singular pattern of substance use. As a result, SDS for a single drug provides a more accurate comparison of SDS scores across different drugs within the specific one-drug group, as SDS scores for multiple drugs may influence each other’s interpretations.
Methods
Participants
This study used data from the (Global Drug Survey Citation2018). The GDS is an annual online anonymous drug survey that has been conducted since 2011 (Winstock et al. Citation2022) Details about the GDS’s methodology, including survey design, recruitment and representativeness have been previously described (Barratt et al. Citation2017; Winstock et al. Citation2022). Participants are eligible to complete the GDS if they are at least 16 years old and have used at least one drug (including alcohol) in the past 12 months. GDS2018 was open for completion between November 6, 2017, and January 10, 2018, and was completed by 130,761 participants from 206 countries. The survey was translated into 18 languages; English, German, Serbian, Czech, Georgian, Azerbaijani, Hebrew, Polish, French, Italian, Spanish (South American Spanish), Portuguese, Flemish, Hungarian, Turkish, Finnish and Danish). For this study, the analysis was restricted to participants who provided responses to at least one of the nine drug categories included in the survey (see below), who answered all five SDS questions for the particular drug and provided a valid response with respects to their age, gender and exercise frequency (N = 57,110). Additionally, we partitioned this sample into two distinct groups. The first group comprises individuals who indicated use of only one of the nine substances in the last 12 months, which forms the primary focus of our analytical models. The second group includes participants who indicated use of one of the nine substances and at least one additional substance (of the nine). Approval for this study was granted by the University College London Research Ethics Committee (11671/001) and ratified by The University of Queensland (No: 2017001452) and the University of New South Wales (HREC HC17769) ethics committees.
Measures
Substance
The substances included in this study are cannabis, MDMA, cocaine, amphetamine (powder and paste), ketamine, methamphetamine, GHB and mephedrone. These were the substances in the GDS2018 survey with associated questions for the Severity of Dependence Scale (SDS).
Severity of dependence scale (SDS)
The SDS is a brief five-item scale that has been shown to effectively measure dependency across various drug types (Gossop et al. Citation1995), demonstrating good test-retest reliability (0.89; Gossop et al. Citation1997). The scale comprised five questions:
Did you ever think your use of [named drug] was out of control?
Did the prospect of not using [named drug] make you very anxious or worried?
Did you ever worry about your use of [named drug]?
Did you ever wish you could stop using [named drug]?
How difficult would you find it to stop or go without [named drug]?
The questions were slightly modified such that stigmatizing language originally used in question 2 “missing a fix (or dose) or not chasing” was changed to “not using.” Each question is rated on a 4-point scale. For questions 1, 2 and 3 the scoring and wording are 0 “Never or almost never,” 1 “Sometimes,” 2 “Often” 3 “Always or nearly always.” For question 3, response options are 0 “Not at all,” 1 “A little,” 2 “Quite a lot,” 3 “A great deal.” For question 5, response options include 0 “Not difficult,” 1 “Quite difficult,” 2 “Very difficult,” 3 “Impossible.” The higher the score, the higher the level of dependency, ranging from 0–15 (Gossop et al. Citation1995).
Exercise frequency
Participants were asked: “How often in the last year did you exercise (i.e., play sport, run, gym, yoga, etc.)?” Seven response options were provided: Never, less than once every 3 months, once a month, once every fortnight, once or twice a week, 3 to 4 times a week, or more than 4 times a week. These responses were reclassified into three categories, in order to simplify the interpretation and conform broadly with previous research (Vina et al. Citation2012) and physical activity recommendations (Wasfy and Baggish Citation2016).
Participants who indicated never exercising, exercising less frequently than once every 3 months, or on a monthly basis were coded as “None or low.” Those reporting exercising either once a fortnight or once or twice a week were coded as “moderate, and those exercising 3–4 times per week or more were coded (“High..”
Data analysis
The analysis employed negative binomial regression to investigate the association between exercise and SDS scores within each drug category; analysis was stratified across the two sample subsets. To compare differences in exercise frequency and SDS scores for each particular drug across the two groups a third model was undertaken including an interaction term between exercise frequency and sample subset (0 for 2 or more drugs; 1 for one drug only). This approach tested whether the pattern of exercise frequency on SDS scores differs by drug relative to respondents reporting using the drug exclusively or using the drug in the presence of other drugs (where we have asked the SDS items).’ For each drug category, the typical α = 0.05 has been applied to test for significance.
Results
The study sample comprises 57,110 participants who indicated using at least one of the nine drugs in the last year and answered the corresponding SDS questions. The majority of respondents identified as men/male (70.5%), followed by women/female (28.4%); approximately 1.1% identified as non-binary or a different identity. The sample’s mean age was 25.3 years (SD = 8.8), ranging from 16 to 85 years (median 23; interquartile range 19–29). Among the entire sample, exercise frequency over the last 12 months varied, with 33.4% indicating low or no exercise, 43.7% reporting moderate exercise, and 22.9% engaging in high exercise frequency (see ).
Table 1. Demographic characteristics and exercise frequency distribution (N = 57,110).
presents socio-demographic characteristics for the sample of respondents who provided SDS information to only one of the nine drugs listed. This subsample (see “Only one drug” in ) consisted primarily of respondents who reported moderate to high frequency of exercise in the past 12 months (68.6%), identified as male (70.4%) with a mean age 25.7 years (SD = 9.8), ranging from 16 to 85 years. Almost 60% of the sample were aged 16–24 years; 6.1% were 45 years of age or older. When considering only those respondents who reported using a single illicit substance (n = 31,862), the distribution was similar, with 31.4% indicating low exercise, 44.2% reporting moderate exercise, and 24.4% engaging in high exercise frequency.
Drug use and SDS scores
Stratified by the two subsamples, presents the prevalence of drug use for each of the nine drugs and a summary of the SDS score and SDS classification categories (mild, moderate and severe) associated with that drug. In the full sample, cannabis was used by 91.1% of respondents in the past year, while mephedrone had the lowest usage at 1.5%. SDS scores showed that for most drugs, the majority of respondents had no to mild dependence (upper-quartile SDS score ≤ 3), but 19.1% to 3.2% were moderate to severe. In the subgroup reporting only one drug, cannabis (91.3%), MDMA (4.0%), and cocaine (3.3%) were most commonly used. Stimulant drugs had higher rates of moderate to severe SDS (cocaine 12.4%, amphetamine powder 13.8%, amphetamine paste 24.1%, methamphetamine 32.8%). GHB (32.3%) and mephedrone (25.0%) also showed elevated rates.
Table 2. SDS scores and classification distribution for different substances.
Exercise and SDS scores
and presents results from the negative binomial regression model for each drug with the single independent categorical variable of exercise frequency. The analyses were stratified for the two samples: respondents who provided SDS responses for the listed drug and at least one other drug (first column series) and respondents who provided SDS responses for where they had only consumed the one listed drug in the past 12 months. The reference category is low exercise. To produce the results in and compare the overall effect of exercise on SDS responses the two groups of data were combined and an interaction term between exercise and group was generated (see Formula 1). Column 3 in presents the χ2 Wald test for the interaction effect of the exercise frequency and group.
Figure 1. Predicted SDS scores by frequency of exercise for the whole sample (reporting SDS for 2 drugs or more) and the subsample (reporting SDS only for the one drug used). Note: results for Amphetamine (paste), GHB and mephedrone are not reported due to low numbers in the subsample.
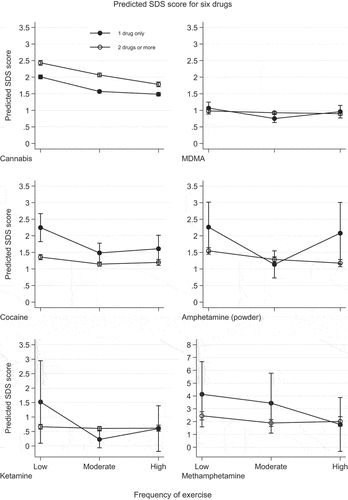
Table 3. Incident rate ratios (IRRs) and 95% confidence intervals (CI) for exercise categories.
, column 1, along with the hollow-circle lines in , highlights the incident rate ratio (IRR; see ) and the predicted SDS score (hollow-circle lines; ). A clear trend emerges for SDS scores when comparing one drug use to multiple drug use. For six of the nine drugs (cannabis, MDMA, cocaine, methamphetamine, amphetamine powder, and paste), exercise frequency significantly associates with lower SDS scores. Specifically, moderate to high exercise is linked to lower SDS scores. However, the impact varies; for example, with MDMA, moderate versus low exercise showed no significant difference (IRR 0.946; 95% CI 0.892–1.002). Conversely, for cocaine, high exercise correlated with higher SDS scores (IRR 1.216; 95% CI 1.069–1.382), and this difference was statistically significant. For ketamine, GHB, and mephedrone, exercise frequency did not significantly affect SDS scores.
In and ‘s second column, IRR and predicted SDS scores demonstrate a consistent trend for individuals reporting single-drug use. Notably, exercise frequency significantly influences SDS scores for five of eight drugs (see Wald test, column 1). More exercise, particularly transitioning from low to moderate or moderate to high, consistently relates to lower SDS scores. This trend holds for cannabis and methamphetamine, with cannabis being statistically significant (p < .001). Similarly, respondents with moderate exercise frequency versus low exhibit decreased SDS scores for MDMA, cocaine, amphetamine (paste and powder), and ketamine. However, high exercise frequency is linked to higher SDS scores for these drugs, with statistical significance for MDMA, cocaine, amphetamine (paste and powder), and ketamine. For mephedrone, with no overall association between exercise frequency and SDS scores, further exploration was unwarranted.
The final column of presents a Wald test comparing exercise frequency and SDS scores between two groups, indicating differences in SDS score patterns across exercise levels (). Significant differences were found for cannabis, MDMA, and amphetamine (powder). For cannabis users, SDS scores were consistently higher across all exercise levels when compared to those using cannabis and at least one other drug, suggesting lower SDS scores for respondents exclusively using cannabis. MDMA (p = .030) and amphetamine (powder; p = .039) showed marginally significant overall Wald test results. illustrates less distinct differences in SDS scores across exercise frequency for MDMA users. No significant difference in SDS scores was observed for those solely using MDMA compared to those using both MDMA and another drug. A difference was observed for amphetamine (powder), with significantly larger predicted SDS scores for low and high exercise frequency users compared to those exclusively using amphetamine (powder) or using it with another drug.
Discussion
This study examined the associations among exercise frequency, substance use, and substance use severity in a large cohort of Global Drug Survey respondents. While the upper-quartile SDS scores for all nine drugs indicated no to mild substance use severity for most participants, the percentage classified as moderately or severely dependent ranged from 19.1% for methamphetamine to 3.2% for ketamine. Participants who reported exclusively using a single drug exhibited higher inter-quartile SDS score ranges for most substances, highlighting potentially divergent substance use patterns in this subgroup.
The hypotheses underpinning this study were twofold: firstly, we postulated that individuals engaging in more frequent exercise would yield lower SDS scores across the gamut of illicit substances; secondly, we anticipated that this association would persist even when examining participants who solely used one illicit substance from the nine considered. The hypotheses of this study were partially supported by the findings. Stratifying by respondents’ use of multiple drugs versus those using only one, results demonstrated a significant inverse relationship between exercise frequency and SDS scores for five of the eight drugs studied among those who reported using a single drug in the past year. Notably, participants reporting moderate to high exercise frequency consistently exhibited lower SDS scores. This effect was statistically significant for cannabis, somewhat supporting the theoretical premise that the coactivation of shared receptors through exercise and endocannabinoid release could potentially reduce substance use severity (Lynch et al. Citation2013; Raichlen et al. Citation2012). In contrast, respondents using substances like MDMA, cocaine, amphetamine (paste and powder), and ketamine displayed differential dynamics: moderate exercise frequency corresponded to lower SDS scores but transitioning to high exercise frequency yielded higher SDS scores, with significance attained for MDMA and cocaine. Given that exercise may help reduce the use of cannabis and stimulants particularly (Buchowski et al. Citation2011; Vidot et al. Citation2019; Zhu et al. Citation2018) the current data partially fit with extant work.
Although prior research has consistently highlighted the positive impact of exercise on various substance use disorders (Barton and Pretty Citation2010; Daley Citation2008; Furzer et al. Citation2021; Pretty et al. Citation2007; Ramadas et al. Citation2021; Wegner et al. Citation2014), the results from this study provide more intricate and considered understanding. Specifically, the results for individuals using multiple drugs demonstrated more nuanced relationships than those from single drug participants. More specifically, exercise frequency showed a significant association with lower SDS scores for cannabis, MDMA, and amphetamine (powder). For MDMA, the difference in SDS scores was not significant when comparing moderate to low exercise frequency. Additionally, different patterns were evident in the dynamics between exercise frequency and SDS scores for different drugs. Notably, people reporting cannabis use reported consistently higher SDS scores across exercise frequency categories when used in conjunction with other drugs, hinting at potential synergistic effects. These findings suggest that exercise frequency may exert varying impacts on different substances in relation to disorder development and warrant further investigation.
Limitations
Given the cross-sectional nature of the study, causal inferences regarding this relationship cannot be drawn. The reliance on self-reporting in the data collection introduces potential biases, as seen in varying levels of truthfulness across substances (Hunt et al. Citation2015). Nevertheless, non-probability sampling through anonymous online surveys, like the GDS, serves as a practical and effective method to collect data on stigmatized behaviors among hard-to-reach populations, such as illicit substance users (Barratt et al. Citation2017) although we acknowledge limitations arising from the absence of data from all regions, such as Asia. Further, we did not fully capture the precise stage of respondents’ substance use disorder severity, which could have implications for the relationship between exercise and substance use. Specifically, individuals in different states of substance use severity may exhibit varied exercise behaviors and mental health outcomes (Juel et al. Citation2017). Our study did not measure exercise intensity, a variable known to impact the positive benefits of exercise (Chan et al. Citation2019). Last, the analysis did not treat respondents’ country of residence as a nested variable to address potential country-level variations in exercise patterns, culture, and drug use. Future research could explore country-level effects on substance use disorders, considering exercise profiles.
Implications
Recent prevalence estimates suggest there are over 200 million individuals who report cannabis use in the last year (UNODC Citation2022) and people who use cannabis are at risk of experiencing harms from high levels of use (Connor et al. Citation2021; Peacock et al. Citation2018). Therefore, it is important to consider interventions which may mitigate these harms. Given that limited research has been conducted specifically on exercise among populations using cannabis (Buchowski et al. Citation2011) the present findings contribute to an important gap. Although some studies (Brellenthin and Koltyn Citation2016; Buchowski et al. Citation2011) have observed reductions in craving among cannabis consumers engaging in exercise, these findings have not been tested in clinical trials. The only randomized controlled trial conducted on exercise for cannabis use focused on high-intensity interval training and found reductions in cue-induced craving, suggesting that time rather than exercise itself played a role in craving reduction (Wilson et al. Citation2018). The findings of this study provide insights into the potential application of exercise messaging as a public health strategy for harm reduction and substance use management. Incorporating exercise as a component of harm reduction initiatives and substance use treatment could offer additional benefits for individuals using cannabis.
Research conducted in populations with methamphetamine use disorders have reported non-statistically significant reductions in methamphetamine use among those in exercise conditions compared to control conditions involving health education or contact control (Rawson et al. Citation2015) to which our current data also add further substantiation. However, findings suggest a dose-response effect of the exercise intervention, with greater session attendance associated with greater reductions in methamphetamine use (Rawson et al. Citation2015). Furthermore, individuals with lower severity of methamphetamine use at baseline tend to maintain higher levels of physical activity post-treatment (Rawson et al. Citation2015). In individuals with cocaine use disorder, preliminary evidence from a randomized controlled trial comparing running, walking, and sitting indicates that exercise may play a role in reducing cocaine use compared to a sedentary condition (De La Garza et al. Citation2016). Additionally, exercise has been associated with significant improvements in cardiorespiratory fitness and mental health outcomes in individuals using methamphetamine (Morris et al. Citation2018). The current data extend on this previous evidence, directing public health messaging to be, potentially, tailored to illuminate the specific benefits of exercise in managing methamphetamine and stimulant use. Doing so provides an opportunity to elevate awareness regarding exercise’s role in harm reduction among individuals navigating the challenges of risky stimulant use.
Conclusions
This study furthers understanding regarding the relationships which may exist between exercise and substance use severity. Transitioning from minimal to regular exercise emerges as a potential means to mitigate the risk of substance use disorders, especially in the context of stimulant drugs and cannabis. However, while the findings suggest potential benefits of exercise in relation to reduced substance use, other factors that may better explain the observed relationships. Therefore, the role of exercise interventions in mitigating substance use disorders requires further investigation through prospective designs and randomized controlled trials.
Acknowledgments
We would like to thank the participants of the Global Drug Survey. We are grateful for the promotion of GDS by a long list of world media partners (see www.globaldrugsurvey.com).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data available from corresponding author on request.
Additional information
Funding
References
- Barratt, M. J., J. A. Ferris, R. Zahnow, J. J. Palamar, L. J. Maier, and A. R. Winstock. 2017. Moving on from representativeness: Testing the utility of the global drug survey. Substance Abuse: Research & Treatment 11:1178221817716391. doi:10.1177/1178221817716391.
- Barton, J., and J. Pretty. 2010. What is the best dose of nature and green exercise for improving mental health? A Multi-Study analysis. Environmental Science & Technology 44 (10):3947–3955. doi: 10.1021/es903183r.
- Basso, J. C., and W. A. Suzuki. 2017. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: A review. Brain Plasticity 2 (2):127–152. doi:10.3233/BPL-160040.
- Boecker, H., T. Sprenger, M. Spilker, G. Henriksen, M. Koppenhoefer, K. Wagner, et al. 2008. The Runner’s high: Opioidergic mechanisms in the human brain. Cerebral Cortex 18 (11):2523–2531. doi:10.1093/cercor/bhn013.
- Brandon, T. H., J. I. Vidrine, and E. B. Litvin. 2007. Relapse and relapse prevention. Annual Review of Clinical Psycholology 3 (1):257–84. doi:10.1146/annurev.clinpsy.3.022806.091455.
- Brellenthin, A. G., and K. F. Koltyn. 2016. Exercise as an adjunctive treatment for cannabis use disorder. The American Journal of Drug and Alcohol Abuse 42 (5):481–489. doi:10.1080/00952990.2016.1185434.
- Buchowski, M., N. Meade, E. Charboneau, S. Park, M. Dietrich, R. Cowan, P. Martin, and A. V. García. 2011. Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. Plos One 6 (3):e17465. doi:10.1371/journal.pone.0017465.
- Castelpietra, G., A. K. S. Knudsen, E. E. Agardh, B. Armocida, M. Beghi, K. M. Iburg, and L. Monasta. 2022. The burden of mental disorders, substance use disorders and self-harm among young people in Europe, 1990–2019: Findings from the Global Burden of Disease Study 2019. The Lancet Regional Health–Europe 16. doi:10.1016/j.lanepe.2022.100341.
- Chan, J. S., G. Liu, D. Liang, K. Deng, J. Wu, and J. H. Yan. 2019. Special issue–therapeutic benefits of physical activity for mood: A systematic review on the effects of exercise intensity, duration, and modality. The Journal of Psychology 153 (1):102–25. doi:10.1080/00223980.2018.1470487.
- Connor, J. P., D. Stjepanović, B. Le Foll, E. Hoch, A. J. Budney, and W. D. Hall. 2021. Cannabis use and cannabis use disorder. Nature Reviews Disease Primers 7 (1):16. doi:10.1038/s41572-021-00247-4.
- Daley, A. 2008. Exercise and depression: A review of reviews. Journal of Clinical Psychology in Medical Settings 15 (2):140–147. doi:10.1007/s10880-008-9105-z.
- De La Garza, R., II, J. H. Yoon, D. G. Thompson-Lake, C. N. Haile, J. D. Eisenhofer, T. F. Newton, and J. J. Mahoney III. 2016. Treadmill exercise improves fitness and reduces craving and use of cocaine in individuals with concurrent cocaine and tobacco-use disorder. Psychiatry Research 245:133–40. doi:10.1016/j.psychres.2016.08.003.
- Desai, S., B. Borg, C. Cuttler, K. M. Crombie, C. A. Rabinak, M. N. Hill, and H. A. Marusak. 2022. A systematic review and meta-analysis on the effects of exercise on the endocannabinoid system. Cannabis and Cannabinoid Research 7 (4):388–408. doi:10.1089/can.2021.0113.
- Ehrman, R., J. Ternes, C. O’Brien, and A. McLellan. 1992. Conditioned tolerance in human opiate addicts. Psychopharmacology 108 (1–2):218–224. doi:10.1007/BF02245311.
- Furzer, B., A. Rebar, J. A. Dimmock, A. More, A. L. Thornton, K. Wright, and B. Jackson, B. Jackson. 2021. Exercise is medicinewhen you enjoy it: Exercise enjoyment, relapse prevention efficacy, and health outcomes for youth within a drug and alcohol treatment service. Psychology of Sport and Exercise 52:101800. doi:10.1016/j.psychsport.2020.101800.
- Garber, C. E., B. Blissmer, M. R. Deschenes, B. A. Franklin, M. J. Lamonte, I. M. Lee, and D. C. Nieman, D. P. Swain. 2011. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Medicine & Science in Sports & Exercise 43 (7):1334–59. doi:10.1249/MSS.0b013e318213fefb.
- Global Drug Survey. 2018. Global drug survey (GDS) key findings report. https://issuu.com/globaldrugsurvey/docs/report-template/4
- Gossop, M., D. Best, J. Marsden, and J. Strang. 1997. Test-retest reliability of the severity of dependence scale. Addiction 92 (3):353–353. doi:10.1111/j.1360-0443.1997.tb03205.x.
- Gossop, M., S. Darke, P. Griffiths, J. Hando, B. Powis, W. Hall, and J. Strang. 1995. The Severity of Dependence Scale (SDS): Psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction 90 (5):607–614. doi:10.1111/j.1360-0443.1995.tb02199.x
- Heijnen, S., B. Hommel, A. Kibele, and L. S. Colzato. 2016. Neuromodulation of aerobic exercise—a review. Frontiers in Psychology 6:1890. doi:10.3389/fpsyg.2015.01890.
- Hunt, D., R. Kling, Y. Almozlino, S. Jalbert, M. Chapman, and W. Rhodes. 2015. Telling the truth about drug use. Journal of Drug Issues 45 (3):314–329. doi:10.1177/0022042615589406.
- Juel, A., C. B. Kristiansen, N. J. Madsen, P. Munk-Jørgensen, and P. Hjorth. 2017. Interventions to improve lifestyle and quality-of-life in patients with concurrent mental illness and substance use. Nordic Journal of Psychiatry 71 (3):197–204. doi:10.1080/08039488.2016.1251610.
- Leasure, J. L., C. Neighbors, C. E. Henderson, and C. M. Young. 2015. Exercise and alcohol consumption: What we know, what we need to know, and why it is important. Frontiers in Psychiatry 6:156. doi:10.3389/fpsyt.2015.00156.
- Linke, S. E., and M. Ussher. 2015. Exercise-based treatments for substance use disorders: Evidence, theory, and practicality. The American Journal of Dug and Alcohol Abuse 41 (1):7–15. doi:10.3109/00952990.2014.976708.
- Lynch, W., A. Peterson, V. Sanchez, J. Abel, and M. Smith. 2013. Exercise as a novel treatment for drug addiction: A neurobiological and stage-dependent hypothesis. Neuroscience & Biobehavioral Reviews 37 (8):1622–1644. doi:10.1016/j.neubiorev.2013.06.011.
- Marlatt, G. A., and D. M. Donovan, Eds. 2005. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. New York: Guilford press.
- Morris, L., J. Stander, W. Ebrahim, S. Eksteen, O. A. Meaden, A. Ras, and A. Wessels. 2018. Effect of exercise versus cognitive behavioural therapy or no intervention on anxiety, depression, fitness and quality of life in adults with previous methamphetamine dependency: A systematic review. Addiction Science & Clinical Practice 13 (1):1–12. doi:10.1186/s13722-018-0106-4.
- Peacock, A., J. Leung, S. Larney, S. Colledge, M. Hickman, J. Rehm, and L. Degenhardt, R. West, W. Hall, P. Griffiths. 2018. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 113 (10):1905–26. doi:10.1111/add.14234.
- Piatkowski, T. M., K. M. White, L. M. Hides, and P. L. Obst. 2020. Australia’s adonis: Understanding what motivates young men’s lifestyle choices for enhancing their appearance. Australian Psychologist 55 (2):156–68. doi:10.1111/ap.12451.
- Pretty, J., J. Peacock, R. Hine, M. Sellens, N. South, and M. Griffin. 2007. Green exercise in the UK countryside: Effects on health and psychological well-being, and implications for policy and planning. Journal of Environmental Planning and Management 50 (2):211–231. doi:10.1080/09640560601156466.
- Raichlen, D., A. Foster, G. Gerdeman, A. Seillier, and A. Giuffrida. 2012. Wired to run: Exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the ‘runner’s high’. Journal of Experimental Biology 215 (8):1331–1336. doi:10.1242/jeb.063677.
- Ramadas, E., M. P. D. Lima, T. Caetano, J. Lopes, and M. D. A. Dixe. 2021. Effectiveness of mindfulness-based relapse prevention in individuals with substance use disorders: A systematic review. Behavioral Sciences 11 (10):133. doi:10.3390/bs11100133.
- Rawson, R. A., J. Chudzynski, L. Mooney, R. Gonzales, A. Ang, D. Dickerson, and J. Penate, B. A. Salem, B. Dolezal, C. B. Cooper. 2015. Impact of an exercise intervention on methamphetamine use outcomes post-residential treatment care. Drug and Alcohol Dependence 156:21–28. doi:10.1016/j.drugalcdep.2015.08.029.
- Roessler, K. 2010. Exercise treatment for drug abuse - A Danish pilot study. Scandinavian Journal of Public Health 38 (6):664–669. doi:10.1177/1403494810371249.
- Saanijoki, T., L. Tuominen, J. J. Tuulari, L. Nummenmaa, E. Arponen, K. Kalliokoski, and J. Hirvonen. 2018. Opioid release after high-intensity interval training in healthy human subjects. Neuropsychopharmacology 43 (2):246–254. doi:10.1038/npp.2017.148.
- Sherman, C. 2017. Impacts of drugs on neurotransmission. Accessed October 10, 2019. https://www.drugabuse.gov/news-events/nida-notes/2017/03/impacts-drugs-neurotransmission.
- Smith, M. A., and W. J. Lynch. 2012. Exercise as a potential treatment for drug abuse: evidence from preclinical studies. Frontiers in Psychiatry 2:82. doi:10.3389/fpsyt.2011.00082.
- Sparling, P. B., A., Giuffrida, D., Piomelli, L., Rosskopf, and A. Dietrich. 2003. Exercise activates the endocannabinoid system. Neuroreport 14 (17):2209–11.
- Sujkowski, A., L. Hong, R. J. Wessells, and S. V. Todi. 2022. The protective role of exercise against age-related neurodegeneration. Ageing Research Reviews 74:101543. doi:10.1016/j.arr.2021.101543.
- Teixeira, P. J., E. V. Carraça, D. Markland, M. N. Silva, and R. M. Ryan. 2012. Exercise, physical activity, and self-determination theory: A systematic review. International Journal of Behavioral Nutrition and Physical Activity 9 (1):1–30. doi:10.1186/1479-5868-9-78.
- United Nations Office on Drugs and Crime. 2022 World drug report 2022. World Drug Report. Accessed July 18, 2023. https://www.unodc.org/unodc/data-and-analysis/world-drug-report-2022.html.
- Vidot, D., C. Rethorst, T. Carmody, M. Stoutenberg, R. Walker, T. Greer, and M. Trivedi. 2019. Acute and long-term cannabis use among stimulant users: Results from CTN-0037 Stimulant Reduction Intervention using Dosed Exercise (STRIDE) randomized control trial. Drug and Alcohol Dependence 200:139–144. doi:10.1016/j.drugalcdep.2019.02.032.
- Vina, J., F. Sanchis‐Gomar, V. Martinez‐Bello, and M. C. Gomez‐Cabrera. 2012. Exercise acts as a drug; the pharmacological benefits of exercise. British Journal of Pharmacology 167 (1):1–12. doi:10.1111/j.1476-5381.2012.01970.x.
- Volkow, N., J. Fowler, G. Wang, R. Baler, and F. Telang. 2009. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 56:3–8. doi:10.1016/j.neuropharm.2008.05.022.
- Wasfy, M. M., and A. L. Baggish. 2016. Exercise dose in clinical practice. Circulation 133 (23):2297–2313. doi:10.1161/CIRCULATIONAHA.116.018093.
- Wegner, M., I. Helmich, S. Machado, A. Nardi, O. Arias-Carrion, and H. Budde. 2014. Effects of exercise on anxiety and depression disorders: Review of meta- analyses and neurobiological mechanisms. CNS & Neurological Disorders - Drug Targets 13 (6):1002–14. doi: 10.2174/1871527313666140612102841.
- Weinstock, J., M. R. Farney, N. M. Elrod, C. E. Henderson, and E. P. Weiss. 2017. Exercise as an adjunctive treatment for substance use disorders: Rationale and intervention description. Journal of Substance Abuse Treatment 72:40–47. doi:10.1016/j.jsat.2016.09.002.
- Wilson, S. D., R. L. Collins, M. A. Prince, and P. C. Vincent. 2018. Effects of exercise on experimentally manipulated craving for cannabis: A preliminary study. Experimental and Clinical Psychopharmacology 26 (5):456. doi:10.1037/pha0000200.
- Winstock, A. R., E. L. Davies, J. A. Ferris, L. J. Maier, and M. J. Barratt. 2022. Using the global drug survey for harm reduction. In J. Matias, A. Soderholm, K. Skarupova, A. Noor, J. Mounteney (Eds.), EMCDDA Insights (Ed.), Monitoring drug use in the digital age: Studies in web surveys, Vol. 26, Lisbon: European Monitoring Centre for Drugs and Drug Addiction.
- Zhu, D., G. Dai, D. Xu, X. Xu, J. Geng, W. Zhu, X. Jiang, and M. Theeboom. 2018. Long-term effects of Tai Chi intervention on sleep and mental health of female individuals with dependence on amphetamine-type stimulants. Frontiers in Psychology 9:9. doi:10.3389/fpsyg.2018.01476.
