Abstract
Objective. To explore the association between abdominal obesity and insulin resistance in patients with type 2 diabetes. Design. A cross-sectional observational study. Setting. Primary care in Skara, Sweden. Subjects. A total of 198 men and 186 women with type 2 diabetes who consecutively completed an annual check-up in 1992–1993. Main outcome measures. Abdominal obesity was defined according to criteria for the metabolic syndrome using the waist circumference (WC): >102 cm for men and >88 cm for women. Insulin resistance was estimated using the Homeostasis Model Assessment (HOMA), and was dichotomized by the 75th percentile (IR). Results. Abdominal obesity was found in 66 men (33%), and in 106 women (57%). Pearson's correlation coefficients between components of the metabolic syndrome and IR were statistically significant for WC, waist–hip ratio, serum triglycerides, and HDL cholesterol, and were higher for WC (0.40) than for waist–hip ratio (0.23) in both genders (p < 0.001). The association between WC and IR was challenged by successively entering other components of the metabolic syndrome into the model in a logistic regression. In the final model, adjusting for differences in age, systolic blood pressure, HbA1c, serum triglycerides, HDL cholesterol, and microalbuminuria, the association remained statistically significant both in men (OR 8.6, 95% CI 3.0–25.2, p < 0.001), and in women (OR 5.6, 95% CI 1.7–18.1, p = 0.004). Conclusions. WC provides a feasible measure for insulin resistance in the vast majority of subjects with type 2 diabetes. It is convenient and less expensive than direct means and could be used as a proxy for insulin resistance in population studies.
Type 2 diabetes is a common condition managed in primary healthcare. Evidence is accumulating that insulin resistance may be the common underlying etiological factor for the individual components of the metabolic syndrome Citation[1–3] and for type 2 diabetes. Abdominal obesity is a strongly concluded phenotypic companion for a cluster of metabolic abnormalities characterized by insulin resistance Citation[4], Citation[5]. However, the association with abdominal obesity and features of the metabolic syndrome has been reported to vary with gender Citation[6], Citation[7], and with different degrees of obesity Citation[8], Citation[9]. Although abdominal obesity is the best obesity-related predictor of type 2 diabetes Citation[10] it is not clear to what extent abdominal obesity can be used as a surrogate measure for insulin resistance in subjects who have already developed type 2 diabetes. The aim of this study was to explore the association between abdominal obesity and insulin resistance in patients with type 2 diabetes in primary care in a Swedish community accounting for other components in the metabolic syndrome.
Type 2 diabetes is a common condition managed in primary healthcare. The relationship between insulin resistance and abdominal obesity is generally well established.
Waist circumference provides a feasible surrogate measure for insulin resistance in the vast majority of subjects with type 2 diabetes.
Waist circumference may be used as a tool to individualize adequate treatment for patients with type 2 diabetes in primary care and for follow-up of such treatment.
Material and methods
Patients with type 2 diabetes who consecutively completed an annual checkup at the hypertension and diabetes outpatient clinic in Skara, Sweden, from June 1992 to September 1993 were eligible for the study. After exclusion of 16 subjects with missing data on waist or hip circumferences, 384 patients with type 2 diabetes (198 men and 186 women) remained for the descriptive characteristics given in . After exclusion of 65 patients who were on insulin treatment and 11 subjects with missing values for insulin, further analyses of the association between insulin resistance and abdominal obesity (see Tables and ) were confined to 163 men and 145 women.
Figure 1. Insulin resistance estimated by the homeostasis model assessment (geometrical mean of HOMA) as a function of waist circumference (centimeter) in 163 men and 145 women with type 2 diabetes.
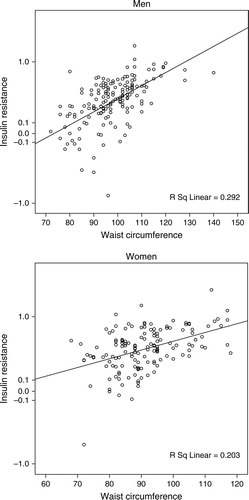
Table I. Descriptive characteristics in male and female patients with diabetes type 2 by subgroups of abdominal obesity defined by waist circumference (p-values for non-ObesityWC vs. ObesityWC explored by logistic regression), Skaraborg Hypertension and Diabetes Project 1992–1993.
Table II. Correlations between HOMA IR and other components of the metabolic syndrome in 163 men and 145 women with type 2 diabetes, Skaraborg Hypertension and Diabetes Project 1992–1993.
Table III. Associations between HOMA IR and abdominal obesity estimated by waist circumference (ObesityWC), in 163 men and 145 women with type 2 diabetes, Skaraborg Hypertension and Diabetes Project 1992–93.
Table IV. Relation between results of waist circumference and HOMA IR in 308 subjects with type 2 diabetes, Skaraborg Hypertension and Diabetes Project 1992–93.
Procedure and laboratory investigations
The study visit was conducted by nurses at the hypertension and diabetes outpatient clinic who were specially trained for this task. The procedure has been described in detail previously Citation[11], Citation[12]. The investigation included a standard medical history and blood specimens were drawn in the morning after a 10 h overnight fast. Routine tests such as fasting blood glucose and HbA1c were analyzed at the local hospital laboratory (Kärnsjukhuset, Skövde, Sweden), and serum lipids at a lipids laboratory (Lund University Hospital, Sweden), respectively. The presence of microalbuminuria in urine was ascertained using a dipstick (Micral®test) Citation[13]. Microalbuminuria was defined as ≥20 µg/l in the first morning sample of urine. Serum insulin was analyzed using a radioimmunoassay with < 0.3% cross-reactivity for proinsulin (Wallenberg Laboratory, Malmö University Hospital, Sweden) Citation[14]. Insulin resistance was assessed from the fasting glucose and insulin concentrations by using the HOmeostasis Model Assessment (HOMA) formula: fasting insulin (µU/ml) x fasting glucose (mmol/l)/22.5 Citation[15]. The model is not applicable to subjects treated with insulin and 65 patients whose treatment included insulin were excluded from the analysis of HOMA. Insulin resistance (HOMA IR) was considered when the HOMA index for insulin resistance exceeded the 75th percentile.
Anthropometric measurements
Weight (to the nearest 0.1 kg), and height as well as waist circumference (WC) and hip circumference (all to the nearest cm), were recorded. Body mass index (BMI) was calculated by the formula weight (kg)/height2 (m2), and waist–hip ratio by dividing waist circumference (cm) by hip circumference (cm). Men with WC > 102 cm, and women with WC > 88 cm were considered to be abdominally obese (obesityWC) according to guidelines of the third Adult Treatment Panel of the National Cholesterol Education Program for identification of individuals with metabolic syndrome Citation[16].
Hypertension and diabetes
Blood pressure was recorded to the nearest 2 mm Hg using a Tricuff® for automatic adjustment of cuff size to arm circumference Citation[17]. Definition of hypertension was based on either pharmacological treatment for hypertension, or at least three consecutive readings of diastolic blood pressure ≥ 90 mm Hg irrespective of systolic blood pressure. The treatment goal was set at diastolic blood pressure ≤90 mm Hg Citation[18]. In 100 men (50%) and 94 women (50%) type 2 diabetes was combined with hypertension, while type 2 diabetes without hypertension was present in 98 men and 92 women. The criteria for diagnosis of diabetes mellitus have followed the World Health Organization recommendations from 1985 Citation[19] and diabetes mellitus was considered as type 1 or type 2 diabetes based on clinical criteria Citation[11]. All current medications for hypertension and diabetes were recorded.
Statistical analysis
The SPSS Base System for Windows 11.5 was used for data analyses. Standard methods were used for descriptive statistics. Confounding from differences in age and sex were accounted for by multivariate analyses or by stratification. Due to skewed distributions of HOMA IR, serum insulin and serum triglycerides geometric means for these variables were used in analyses.
Correlations between HOMA IR and components of the metabolic syndrome were analyzed with bivariate correlation accounting for differences in age and are presented as Pearson's correlation coefficients. Differences between groups in continuous variables were analyzed with analysis of covariance. Associations between categorical variables were estimated by logistic regression and presented as odds ratios (OR) with 95% confidence intervals (CI). The association between abdominal obesity by WC (obesityWC) and HOMA IR was further challenged by adjustment for differences in components of the metabolic syndrome by successively entering them as covariates in the model one by one. All tests were two-sided and statistical significance was assumed when p < 0.05.
Ethics
The Skaraborg Hypertension and Diabetes Project has been approved by the Committee on Research Ethics at the Medical Faculty of the University of Göteborg.
Results
Descriptive characteristics in male and female patients with type 2 diabetes in subgroups of abdominal obesity (obesityWC) are given in . Levels of HDL cholesterol were lower and BMI, waist–hip ratio, serum insulin, and HOMA IR higher in both men and women with abdominal obesity compared with their contrasts. In men, abdominal obesity was also associated with higher levels of serum triglycerides compared with men without abdominal obesity. There were no significant differences in fasting blood glucose, HbA1c, total cholesterol, or LDL cholesterol in either sex.
gives the correlations between HOMA IR and components of the metabolic syndrome. The correlations were statistically significant for WC, waist–hip ratio, serum triglycerides, and inversely correlated to HDL cholesterol. Pearson's correlation coefficients were slightly higher for WC than for hip circumference followed by waist–hip ratio in both genders. Further analyses were thus focused on WC as measure for abdominal obesity.
As can be seen in the associations between obesityWC and insulin resistance were highly significant in both sexes. In both genders, there was a significant crude linear association between WC and insulin resistance as estimated by the HOMA index (). It was challenged by entering other components of the metabolic syndrome into the model (). In the final model, in , adjusting for differences in age, systolic blood pressure, HbA1c, serum triglycerides, HDL cholesterol, and microalbuminuria, the association remained statistically significant both in men (OR 8.6, 95% CI 3.0–25.2, p < 0.001), and in women (OR 5.6, 95% CI 1.7–18.1, p = 0.004). In this final model, only WC was associated with HOMA IR in men while in women WC, systolic blood pressure (p = 0.013) and serum triglycerides (p = 0.049) remained statistically significant.
The sensitivity and specificity for obesityWC were 0.76 and 0.67, respectively, based on the figures given in .
Discussion
There was a strong association between abdominal obesity and insulin resistance in this community-based sample of 308 subjects with type 2 diabetes that was stronger for waist circumference than for waist–hip ratio. It was very consistent within the study population and independent of other components of the metabolic syndrome.
We did not measure insulin resistance directly in this study. However, HOMA IR is based on fasting insulin and fasting glucose and has been validated against direct methods previously and has been shown to correlate well Citation[20]. Furthermore, the HOMA method has also been shown to correlate well to direct methods in subjects with various degrees of glucose tolerance including patients who have already developed diabetes Citation[21]. The 75th percentile of the HOMA index for insulin resistance was used as cut-off to identify those patients with type 2 diabetes who would be relatively best characterized as insulin resistant to further explore associations with other components of the metabolic syndrome. This should be recognized as different from the more absolute level of insulin resistance referred to in the World Health Organization definition recommended for population studies Citation[1].
Body mass index is the most widely used measure of body size, and is frequently used to estimate the prevalence of obesity within a population Citation[22]. However, this measure does not account for variation in body fat distribution and abdominal fat mass, which can vary substantially within a narrow range of BMI. Excess intra-abdominal fat is associated with greater risk of obesity-related morbidity than is overall adiposity. Thus, measurements of WC and waist–hip ratio have been considered as alternatives to body mass index when exploring the risks associated with obesity. Waist circumference has been shown to be the best simple measure of both intra-abdominal fat mass and total fat Citation[23]. The relationship between insulin resistance and obesity, and abdominal obesity in particular, is generally well established Citation[24]. However, in patients who have already developed type 2 diabetes we have only found one previous publication on this topic from Trinidad, West Indies Citation[25]. In this study, we revealed significant associations in a population of Caucasians with type 2 diabetes with a very consistent pattern remaining in subgroups of the components included in the metabolic syndrome.
The correlation between HOMA IR and dyslipidemia (high serum triglycerides and low HDL cholesterol) is illustrated in the correlation analyses in . These findings highlighting insulin resistance, WC, and dyslipidemia are consistent with previous studies on the metabolic syndrome using factor analyses where insulin resistance, body mass, and dyslipidemia, particularly high serum triglycerides, appear to represent a composite central metabolic factor Citation[26]. Our study thus confirms the close association between body mass, insulin resistance, and dyslipidemia within the metabolic syndrome. The occurrence of this central metabolic factor thus seems to be valid also in a population where type 2 diabetes has already developed, and we accordingly conclude that WC should be used as a marker for the core of the metabolic syndrome.
Type 2 diabetes is a heterogeneous condition entailing subcategories characterized by different pathogenetic pathways Citation[11], Citation[27]. Many cases are related to insulin resistance in the metabolic syndrome, while others would rather follow a primary failure of insulin secretion from the beta-cells Citation[28]. Telling the difference between these categories would be clinically important as it would help in understanding the risk-factor pattern, individualizing the treatment, and estimating the risk of future vascular complications Citation[29]. Furthermore, this is also important since there is accumulating evidence that overweight subjects with type 2 diabetes with features characterizing insulin resistance should preferably be assigned to treatment that decreases insulin resistance, such as metformin, or maybe PPARγ agonists Citation[30]. Even though the sensitivity and specificity were rather low, measuring WC may also be a good tool for follow-up of such treatment and to follow changes in individual patients. However, WC is often ignored in follow-up of type 2 diabetes and according to unpublished data from Sweden, 2004, WC is measured by a nurse or physician in less than 1% of patients with type 2 diabetes (personal communication: Jan Cederholm at National Diabetes Register).
We conclude that abdominal obesity measured by waist circumference provides a good measure for insulin resistance in this ethnically homogenous primary care population that included the vast majority of people with type 2 diabetes in a geographically defined area. Furthermore, waist circumference is a convenient and feasible surrogate measure for insulin resistance in subjects with type 2 diabetes in primary healthcare and is less expensive than direct means.
This study was supported by grants from the Swedish Research Council, Skaraborg Institute, The Health & Medical Care Committee of the Regional Executive Board of the Region Västra Götaland, Skaraborg Primary Care, the NEPI Foundation (The Swedish Network for Pharmacoepidemiology), and the Faculty of Medicine, Lund University.
References
- Alberti KG, Zimmet PZ, for the WHO Consultation Group. Definition, diagnosis and classification of diabetes and its complication. Diabet Med 1998;15:539–53.
- Zimmet PZ. Kelly West Lecture 1991. Challenges in diabetes epidemiology: From the west to the rest. Diabet Care 1992; 15: 232–52
- Stern MP. The insulin resistance syndrome. International textbook of diabetes mellitus2nd edn, KGMM Alberti, PZ Zimmet, RA DeFronzo. Wiley, Chichester 1997; 255–83
- Groop L. Genetics of the metabolic syndrome. Br J Nutr 2000; 83: 39–48
- Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiological Rev 1994; 74: 761–811
- Snehalatha C, Sivasankari S, Satyavani K, Vijay V, Ramachandran A. Insulin resistance alone does not explain the clustering of cardiovascular risk factors in southern India. Diabet Med 2000; 17: 152–7
- Ho SC, Chen YM, Woo JL, Leung SS, Lam TH, Janus ED. Association between simple anthropometric indices and cardiovascular risk factors. Int J Obes Relat Metab Disord 2001; 25: 1689–97
- Daniel M, Marion SA, Sheps SB, Hertzman C, Gamble D. Variation by body mass index and age in waist-to-hip ratio associations with glycemic status in an aboriginal population at risk for type 2 diabetes in British Columbia, Canada. Am J Clin Nutr 1999; 69: 455–60
- Ascaso JF, Romero P, Real JT, Lorente RI, Martinez-Valls J, Carmena R. Abdominal obesity, insulin resistance, and metabolic syndrome in a southern European population. Eur J Intern Med 2003; 14: 101–6
- Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994; 17: 961–9
- Ostgren CJ, Lindblad U, Bog-Hansen E, Ranstam J, Melander A, Rastam L. Differences in treatment and metabolic abnormalities between normo- and hypertensive patients with type 2 diabetes: The Skaraborg Hypertension and Diabetes Project. Diabet Obes Metab 1999; 1: 105–12
- Bog–Hansen E, Merlo J, Gullberg B, Melander A, Rastam L, Lindblad U. Survival in patients with hypertension treated in primary care: A population-based follow-up study in the Skaraborg Hypertension and Diabetes Project. Scand J Prim Health Care 2004; 22: 222–7
- Spooren PF, Lekkerkerker JF, Vermes I. Micral-Test: A qualitative dipstick test for microalbuminuria. Diabetes Res Clin Pract 1992; 18: 83–7
- Andersen L, Dinesen B, Jorgensen PN, Poulsen F, Roder ME. Enzyme immunoassay for intact human insulin in serum or plasma. Clin Chem 1993; 39: 578–82
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–19
- Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults: Executive summary of the third report of the national cholesterol education program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment Panel III). JAMA 2001;285:2486–97.
- Rastam L, Sjonell G. A new device for measuring blood pressure in adults. Lancet 1991; 337: 249–50
- National Board of Health and Welfare Drug Information Committee. Treatment of mild hypertension. Stockholm; 1987.
- World Health Organization Expert Committee. Diabetes mellitus: Technical Report. Geneva: WHO; 1985. p 742.
- Haffner S, Miettinen H, Stern M. The homeostasis model in the San Antonio Heart Study. Diabetes Care 1997; 20: 1087–92
- Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2002; 25: 626–40
- Colditz G, Willett W, Rotnitzky A, Manson J. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995; 122: 481–6
- Valsamakis G, Chetty R, Anwart A, Banarjee K, Barnett A, Kumar S. Association of simple anthropometric measures of obesity with visceral fat and the metabolic syndrome in male Caucasian and Indo-Asian subjects. Diabet Med 2004; 21: 1339–45
- Despres JP. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition 1993; 9: 452–9
- Ezenwaka CE, Offiah NV. Abdominal obesity in type 2 diabetic patients visiting primary healthcare clinics in Trinidad, West Indies. Scand J Prim Health Care 2002; 20: 177–82
- Lindblad U, Langer RD, Wingard DL, Thomas RG, Barrett-Connor EL. Metabolic syndrome and ischemic heart disease in elderly men and women. Am J Epidemiol 2001; 153: 481–9
- Groop LC. The etiology of non-insulin-dependent diabetes mellitus. In: Leskie RDG, editor. Molecular pathogenesis of diabetes mellitus. Front Horm Res 22, 131–56. Basel: Karger; 1997.
- Tuomi T, Groop L, Zimmet PZ, Rowley MJ, Knowles W, Mackay R. Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non-insulin-dependent onset of disease. Diabetes 1993; 42: 359–62
- Hansen LJ, Olivarius Nde F, Siersma V, Drivsholm T, Andersen JS. Individualised treatment goals in diabetes care. Scand J Prim Health Care 2004; 22: 71–7
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005; 366: 1279–89