Abstract
Objectives. To examine determinants of bleeding complications during warfarin treatment in an unselected patient population and evaluate possible differences in safety between specialized anticoagulation clinics and primary healthcare centres. Design. Prospective cohort study. Data were collected with an admission form and medical records were scrutinized in order to pursue all adverse events. Differences between groups were estimated with a t-test and chi-squared test, and univariate and multivariate Cox regression analysis. Setting. All patients treated and monitored with oral anticoagulation in primary healthcare centres and specialized anticoagulation clinics in the Sundsvall and Skellefteå region (northern Sweden) during a five-year period. Subjects. A total of 2731 patients corresponding to 5044 treatment years. Main outcome measures. Bleedings were classified as fatal or major. Major bleedings were defined as an event causing admission, prolonged in-hospital care or death. Results. In total 195 major bleedings occurred corresponding to 3.9% per treatment year, including 34 fatal events (0.67% per treatment year). Patients monitored at the two specialized anticoagulation clinics combined had a major bleeding frequency of 4.1% as compared with 3.9% at primary healthcare units. The frequency of fatal haemorrhage was 0.57% and 0.76%, respectively. The rate of major and fatal bleeding was age related with an increase of 4% and 5%, respectively, per year. Conclusions. There was no difference in bleeding complications between patients monitored at primary healthcare centres and specialized anticoagulation clinics. Age was continuously and independently associated with bleeding risk. These study data indicate the need to exercise caution in treatment of the elderly.
There has been rapidly expanding use of oral anticoagulants following studies showing the efficacy of oral anticoagulants in atrial fibrillation and after myocardial infarction Citation[1], Citation[2]. High prevalence figures of treatment and costs are observed in primary healthcare Citation[3], Citation[4]. Treatment-related complications are inevitable and lead to both substantial morbidity and expenses Citation[5]. The important question of whether the benefit of oral anticoagulants outweighs the risk of bleeding is the focus in several studies Citation[6–12]. However, in clinical trials there is patient selection and treatment duration is limited. In clinical practice patients are treated for long periods and at high age Citation[13]. Little is known about treatment safety in primary healthcare Citation[13]. The aim of the present study was to examine bleeding complications in an unselected patient population and evaluate possible differences in safety between specialized anticoagulation clinics and primary healthcare centres.
Current opinion suggests that warfarin-treated patients should be monitored at specialized anticoagulation clinics for safety reasons.
In the present study there was no difference in bleeding complications between patients monitored at primary healthcare centres versus specialized anticoagulation clinics.
Age was continuously and independently associated with increased bleeding risk.
Gastrointestinal haemorrhage was the most frequent cause of major bleeding and a history of peptic ulcer disease was a strong risk factor for this complication.
Material and methods
Study design and study population
This prospective cohort study, with the aim of including all patients treated with warfarin, comprises 2731 patients from two different regions in northern Sweden. The Sundsvall region has 130 000 inhabitants and the Skellefteå region has 80 000 inhabitants. The prevalence of patients on oral anticoagulation was 0.7% and 0.6%, respectively. All patients treated with warfarin were registered at baseline, and new patients were then registered consecutively. The maximum follow-up time was 4 years and the mean follow-up time was 1.8 years in the 2731 patients included in this study (1152 women and 1579 men). There were 1194 patients monitored in 18 primary healthcare centres and 1537 at two specialized anticoagulation clinics. In the Sundsvall region, 80% were monitored in primary healthcare units and 20% at an anticoagulation clinic. In the Skellefteå region, 10% were monitored in primary healthcare units and 90% at an anticoagulation clinic. This difference was due to local tradition and we deliberately chose one region with a high and one region with a low proportion monitored in primary healthcare units.
Data collection
The admission form consisted of demographic data, indication for anticoagulation, and intended treatment period. In cases where the indication for treatment was a combination of disorders one principle indication was chosen in the following rank order in descending priority: prosthetic heart valve, venous thromboembolism, atrial fibrillation with and without embolism, other heart disease, ischaemic cerebrovascular disease, peripheral vascular disease, and miscellaneous. In the Sundsvall region the admission form also included comorbid diseases, specifically hypertension, diabetes mellitus, history of peptic ulcer disease, and cancer.
All patients treated and monitored at the specialized clinics or at the primary healthcare units for any time interval during the study period were included in the present study. In both the Sundsvall and Skellefteå regions there are no other sites monitoring warfarin treatment. At each monitoring visit the INR, warfarin dose, adverse effects (including any bleeding event) and next control date were registered. The maximum INR control interval was 35 days. The therapeutic interval for all indications was INR 2.0–3.0.
In an effort to pursue and classify all adverse events the medical records from all warfarin-treated patients during the study period were extensively scrutinized for any adverse event. Only one patient was lost to follow-up. Thus, 99.94% of all records were available for review. Bleeding complications were classified as fatal or major, the latter being defined as a bleeding event causing admission or prolonged in-hospital care and all fatal bleedings.
In 922 cases the duration of anticoagulation treatment was 6 months or less while 793 patients were treated during the whole observation period. In total, 366 patients died during the observation period. Recorded causes of death were acute myocardial infarction or heart failure 189, stroke 62 (including intracerebral haemorrhage in 21), cancer 39, infirmity due to old age 15, respiratory insufficiency 11, pneumonia in 10, gangrene 9, ruptured aortic aneurysm 7, pulmonary embolism 6, peritonitis 4, vasculitis 3, gastrointestinal bleeding 4, septicaemia 2, pancreatitis 1, cholecystitis 1, azotemia 1, liver cirrhosis 1, and unknown reason 1.
Statistical analyses
Differences between groups were assessed by t-test and chi-squared test. Univariate and multivariate Cox regression analysis were performed by the use of the SPSS software version 10.0 (SPSS Inc., Chicago, Ill., USA) and odds ratio (OR) and 95% confidence interval (95% CI) were calculated.
Ethical considerations
The study was approved by the ethical committee of Umeå University and the computer register according to the Swedish governmental rules.
Results
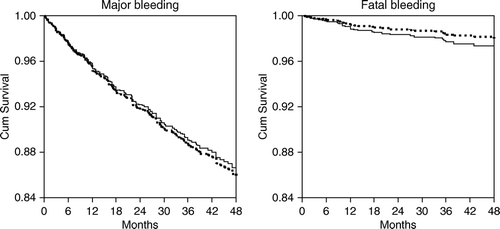
After a total of 5044 treatment years, 195 major bleedings occurred of which 34 were fatal. The risk of major bleeding was 7.1% per treated patient corresponding to a bleeding risk of 3.9% per treatment year (0.67% fatal). Patients monitored in the primary healthcare units had a fatal bleeding frequency of 0.76% (2752 treatment years) as compared with 0.57% (2292 treatment years) at the two anticoagulation clinics (). This difference was not significant with OR = 0.71 (95% CI 0.35–1.43) in univariate analysis and 0.85 (95% CI 0.41–1.76) after adjustment for age, sex, and warfarin indication. The patients monitored at the primary healthcare units had a 3.9% annual risk of major bleeding compared with 4.1% for the group monitored at the anticoagulation clinics, corresponding to an OR = 1.04 (95% CI 0.78–1.39) in univariate Cox regression analysis (). After adjustment for age, sex, and warfarin indication the OR increased to 1.27 (95% CI 0.94–1.71).
Figure 1. Cox regression analysis showing proportion free from major bleeding and fatal bleeding. Dotted lines denote specialized anticoagulation clinics and closed lines denote primary healthcare units.
The mean age of the patients monitored at the anticoagulation clinics was 5 years younger than that of the primary healthcare patients. Indication for anticoagulation was more often venous thromboembolism, atrial fibrillation without previous emboli, heart valve prosthesis, and other heart disease for patients monitored via specialized sites while the primary healthcare centres more frequently monitored patients with atrial fibrillation and previous emboli or with ischaemic cerebrovascular disease (). There were approximately 40% women in both groups.
Table I. Baseline characteristics for patients monitored at specialized anticoagulant clinics and primary healthcare units.
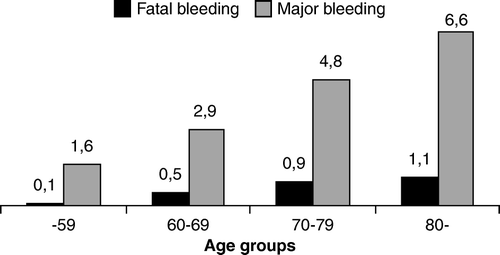
The rate of bleeding increased with age; for major bleeding OR = 1.04 (95% CI 1.03–1.06) and for fatal bleeding OR = 1.05 (95% CI 1.01–1.09) annually. The annual rates of bleeding in different age categories are shown in . In patients 80 years or older the risk of major bleeding was four times higher with OR = 3.97 (95% CI 2.19–7.21) and the risk of fatal bleeding nine times higher with OR = 9.27 (95% CI 1.16–74.16) compared with the group younger than 60 years. In a multivariate Cox regression model in the subgroup of 1579 patients monitored in the Sundsvall region, age as a continuous variable and previous peptic ulcer disease were independently related to bleeding risk (), while gender, diabetes, hypertension, and cancer were not. Of the 58 patients with a history of previous peptic ulcer disease in this subgroup, 12 major and 1 fatal bleeding occurred, all of them from gastrointestinal sources. The risk of gastrointestinal haemorrhage on oral anticoagulation in a patient with a history of previous peptic ulcer was highly significant, OR = 13.4 (95% CI 6.6–27.3), corresponding to a bleeding risk of 12.1 per 100 treatment years.
Table II. Multivariate Cox regression analysis of possible determinants of bleeding (114 events) in the Sundsvall subgroup (n = 1579).
The most frequent indication for warfarin treatment was venous thromboembolism followed by atrial fibrillation in patients with cerebral embolism (). There was a significantly increased risk of major bleeding in patients with venous thromboembolism compared with those with atrial fibrillation with OR = 1.61 (95% CI 1.10–2.37) after adjustment for age and sex. There was no difference in bleeding risk between any of the other indication groups. The main cause of major bleedings was gastrointestinal sources (35%) followed by various haematomas (15%). Among fatal events, intracranial bleeding was the most common (62%). Ruptured aortic aneurysm was a conspicuous cause (21%). The various types of bleeding events are listed in .
Table III. Frequencies of major and fatal bleeding according to indications for oral anticoagulation.
Table IV. Site of major and fatal bleeding events.
Total days in hospital due to bleeding complications were 1858, with a mean hospitalization time of 8.5 days. In patients with major bleeding events the INR was < 2.0 in 20%, between 2.0 and 3.0 in 44%, and greater than 3.0 in 36%. In comparison, patients with fatal bleedings displayed INR <2.0 in 12%, between 2.0 and 3.0 in 69%, and >3.0 in 19%.
Discussion
Previous clinical trials investigating annual bleeding frequency during oral anticoagulation therapy demonstrated ranges from 1.7% to 3.0% for major and approximately 0.6% for fatal bleedings Citation[6], Citation[14]. Clinical trials are confounded by varying degrees of selection bias in the study populations.
Randomized studies have reported frequencies of less than half that described in observation studies, which is probably related to differences in the entry criteria for patients Citation[6]. In a large Italian inception-cohort study, 1.1% major and 0.25% fatal bleeding rates were found in 2745 patients during 2011 patient years Citation[8]. In our study, comprising 2731 patients with over 5044 treatment years, the risk of bleeding was approximately 4 times higher (3.9%). Here all patients on treatment were recorded and all patient records reviewed, while in the Italian study patients with expected difficulty in obtaining appropriate follow-up were excluded. Furthermore, the mean age in our study was 69 years and 56% of the patients were older than 70 years, compared with a mean of 63 years and 35% older patients in the Italian study. In some studies, age was considered a risk factor for bleeding, Citation[6], Citation[8], Citation[9], Citation[15] while others could not find such a relationship Citation[16].
Due to the long observational period and large cohort in this study, the greater risk of bleeding conferred by older age is highly significant and this association was independent of other possible determinants studied. The age and sex distribution in our study reflects the general population on oral anticoagulants in Sweden and, therefore, is relevant for clinicians and their administration of warfarin treatment Citation[20].
In Sweden, warfarin treatment is monitored in both specialized anticoagulation clinics and primary healthcare centres. One topic under debate is whether the skill of the monitoring site has an impact on treatment safety as related to bleeding complications Citation[17–19].
In our study we could not find any advantage with regard to bleeding complications in the specialized clinics. The target INR (2.0–3.0) was the same in both settings. High INR has been suggested to confer a greater risk of bleeding Citation[8], Citation[19–21], while low INR reduces the risk Citation[6]. The majority of major (64%) and fatal bleedings (81%) occurred at therapeutic or subtherapeutic INR levels (i.e. below 3.0). Thus, there are other factors contributing to increased bleeding susceptibility that need to be elucidated. It has also been suggested that the bleeding risk is higher during the first period of warfarin treatment Citation[8]. Due to the non-inceptive design of this study, we can not evaluate this issue as a possible contributor to the increased risk of haemorrhage in patients treated for venous thromboembolism. The mean duration of treatment was shorter in this group (1.08 years) compared with the group with atrial fibrillation (2.04 years). However, differences in frequency of bleeding complications in different indications may also have been influenced by how patients with high-risk features for bleeding complications are dealt with. Treatment with oral anticoagulants may have been withheld in atrial fibrillation while in venous thromboembolism the treatment time has instead been reduced and in patients in need of a heart valve prosthesis a biological prosthesis has been implanted instead of a mechanical prosthesis.
An association with bleeding has been reported for hypertension Citation[11] and cancer Citation[6]. In addition, a history of peptic ulcer disease was reported as a risk factor Citation[6]. In the multivariate model we confirmed the observation for a history of previous peptic ulcer disease as an independent determinant with a threefold increased risk, but no association with hypertension, diabetes, or cancer was found. Moreover, patients with a history of previous peptic ulcer disease had a very high risk of gastrointestinal bleeding during oral anticoagulation, the risk being 13 times higher with an absolute risk of 12.1 per 100 treatment years. These patients must be carefully examined before the initiation of oral anticoagulation. Eradication of helicobacter pylori could possibly diminish this extremely high bleeding risk, although this merits further studies.
Even though oral anticoagulation is beneficial in many situations, bleeding complications constitute a severe clinical problem and may outweigh the benefit of warfarin. The site of INR monitoring seems less important. The data from this study indicate a need for caution in treatment of the elderly. The clinician's reluctance to treat the oldest patients with warfarin when faced with less aggravating indications is hereby supported.
Conflict of interest statement
None of the authors has any conflict of interest to declare.
Acknowledgements
This research was supported by grants from the Joint Committee of Northern Sweden Health Care Region, Västerbotten and Västernorrland counties, and Foundation of Medical Research in Skellefteå.
References
- Petersen P, Bousen G, Gotfredsen J, Andersen E, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. Lancet 1989; 8631: 175–9
- Anticoagulants in the Secondary Prevention of Events in Coronary Thrombosis (ASPECT) Research Group. Effect of longterm oral anticoagulant treatment on mortality and cardiovascular morbidity after myocardial infarction. Lancet 1994;343:499–503.
- Nilsson GH, Bjorholt I, Johnsson H. Anticoagulant treatment in primary health care in Sweden – prevalence, incidence and treatment diagnosis: A retrospective study on electronic patient records in a registered population. BMC Fam Pract 2003; 4: 3
- Hallinen T, Martikainen JA, Soini EJ, Souminen L, Aronkyto T. Direct costs of warfarin treatment among patients with atrial fibrillation in a Finnish health care setting. Curr Med Res Opin 2006; 22: 683–92
- Fanikos J, Grasso-Correnti N, Shah R, Kucher N, Goldhaber SZ. Major bleeding complications in specialized anticoagulation service. Am J Cardiol 2005; 96: 595–8
- Landefeld C, Beyth R. Anticoagulant-related bleeding: Clinical epidemiology, prediction, and prevention. Am J Med 1993; 95: 315–28
- Fang MC, Go AS, Hylek EM, Chang Y, Henault LE, Jensvold NG, Singer DE. Age and the risk of warfarin-associated hemorrhage: The anticoagulation and risk factors in atrial fibrillation study. J Am Geriatr Soc 2006; 54: 1231–6
- Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: An inception-cohort, prospective collaborative study (ISCOAT). Lancet 1996; 348: 423–8
- Torn M, Bollen WL, van der Meer FJ, van der Wall EE, Rosendaal FR. Risks of oral anticoagulant therapy with increasing age. Arch Intern Med 2005; 165: 1527–32
- Beyth R, Landefeld C. Anticoagulants in older patients. Drugs & Aging 1995; 6: 45–54
- Berwaerts J, Webster J. Analysis of risk factors involved in oral-anticoagulant-related intracranial haemorrhages. Q J Med 2000; 93: 513–21
- Lighttowlers S, McGuire A. Cost-effectiveness of anticoagulation in nonrheumatic atrial fibrillation in the primary prevention of ischemic stroke. Stroke 1998; 29: 1827–32
- Jansson J, Westman G, Boman K, Nilsson T, Norberg B. Oral anticoagulant treatment in a medical care district: A descriptive study. Scand J Prim Health Care 1995; 13: 268–74
- Kalra L, Yu G, Perez I, Lakhani A, Donaldson N. Prospective cohort study to determine if trial efficacy of anticoagulation for stroke prevention in atrial fibrillation translates into clinical effectiveness. BMJ 2000; 320: 1236–9
- Perrero PP, Willoughby DF, Eggert JA, Counts SH. Warfarin therapy in older adults: Managing treatment in the primary care setting. J Gerontol Nurs 2004; 30: 44–54
- White R, McKittrick T, Takakuwa J, Callahan C, McDonell M, Fihn S. Management and prognosis of life-threatening bleeding during warfarin therapy. Arch Intern Med 1996; 156: 1197–201
- Johnsson H. Anticoagulant therapy can be safer: An active follow-up and written care programs reduce the number of complications [in Swedish]. Läkartidningen 1999; 96: 3388–90
- Bond C, Raehl C. Pharmacist-provided anticoagulation management in United States hospitals: Death rates, length of stay, Medicare charges, bleeding complications, and transfusions. Pharmacotherapy 2004; 24: 953–63
- Wandell PE. Anticoagulant treatment of patients in Swedish primary health care: Safety aspects. Eur J Clin Pharmacol 2001; 57: 61–4
- Panneerselvam S, Baglin C, Lefort W, Baglin T. Analysis of risk factors for over anti-coagulation in patients receiving long-term Warfarin. Br J Hematol 1998; 103: 422–4
- Kucher N, Connolly S, Beckman J, et al. International normalized ratio increase before warfarin-associated hemorrhage: Brief and subtle. Arch Intern Med 2004; 164: 2176–9