Abstract
Objective. To explore risk factors for acute myocardial infarction (AMI) mortality in hypertensive patients treated in primary care. Design. Community-based cohort study. Setting. Hypertension outpatient clinic in primary health care. Subjects. Patients who consecutively underwent an annual follow-up during 1992–1993 (n =894; 377 men and 517 women). Methods. All events of fatal AMI were ascertained by record linkage to the National Mortality Register to December 31, 2002. Gender-specific predictors for AMI mortality were analysed by Cox regression. Main outcome measure. AMI mortality. Results. During a mean follow-up of 8.7 years 32 cases (8.5%) of fatal AMI were observed in men and 31 cases (6.0%) were observed in women. Most important predictors for AMI mortality in men were microalbuminuria (HR 3.8, CI 1.8–8.0) and left ventricular hypertrophy (HR 4.0, CI 1.7–9.4), whilst in women type 2 diabetes (HR 4.8, CI 2.4–9.8) was an important predictor. In hypertensive patients without diabetes male gender was associated with high AMI mortality (HR 2.7, CI 1.4–5.3), but in patients with both hypertension and type 2 diabetes the higher risk in men disappeared (HR 0.8, CI 0.4–1.7). Conclusion. Cardiovascular disease risk factors remain strong predictors of AMI mortality in hypertensive patients but with a different pattern in the two genders. Markers of organ damage are more important predictors in men, whereas markers of impaired glucose metabolism are more important predictors in women.
Cardiovascular disease risk factors are known to cluster in hypertensive patients Citation[1], Citation[2]. As the existence of other risk factors in hypertensive patients is known to considerably increase morbidity and mortality Citation[3], the importance of identifying coexisting risk factors has repeatedly been emphasized in both national and international guidelines Citation[4–6]. The guidelines conclude that the initiation of blood pressure treatment, as well as the choice of treatment, should be based on a total risk factor evaluation Citation[4–6]. In order to supply additional evidence for these recommendations it is important to identify the risk factors that confer the highest risk of AMI mortality.
In this study the impact of cardiovascular disease risk factors on AMI mortality was explored in a population-based sample of patients with known hypertension treated in primary care with an emphasis on gender differences.
Material and methods
Skaraborg Hypertension Project
When the Skaraborg Hypertension Project was launched in 1977, a special outpatient clinic with appropriately educated nurses was established in primary care in the community of Skara. This project has previously been described in detail Citation[7]. From June 1992 to September 1993 all patients who participated in an annual control at the hypertension and diabetes outpatient clinic in Skara were surveyed for cardiovascular disease risk factors Citation[2]. The Regional Ethical Review Board of the Medical Faculty, Göteborg University, approved the study protocol.
Male gender is generally associated with a higher AMI mortality than female gender. In hypertensive patients without diabetes male gender is a strong predictor of AMI mortality, but in hypertensive patients with known type 2 diabetes there is no gender difference in AMI mortality.
Cardiovascular disease risk factors predict AMI mortality in hypertensive patients but with a different pattern in the two genders. Markers of organ damage are more important predictors in men, whereas markers of impaired glucose metabolism are more important predictors in women.
General practitioners should be observant regarding the high risk of AMI mortality in hypertensive women with diabetes and in hypertensive men with microalbuminuria.
Study population
Skara is a small town in a rural area in the south-west of Sweden. As only one healthcare centre, and no hospital, is available in the town, practically all hypertensive patients in Skara are known at the healthcare centre. Since the start of the Skaraborg Hypertension Project the routines at the healthcare centre have been very strict regarding control of hypertensive patients. The standard routine was that every patient with a diagnosis of hypertension should attend an annual control at the hypertension and diabetes outpatient clinic.
All men and women with known hypertension who participated in the survey were consecutively included in the present analysis. Subjects with type 1 diabetes (n = 33) were excluded. Informed consent was collected from all participants.
Methods
Previous cardiovascular disease (CVD) was defined as previous admission to hospital for stroke or myocardial infarction, or doctor's diagnosis of angina pectoris Citation[8]. Microalbuminuria was considered to be present if albumin concentration was at least 20 mg L−1 in morning urine. Insulin resistance was calculated by homeostasis model assessment (HOMA), using fasting glucose and fasting insulin levels (insulin resistance = fasting insulin (µU ml−1)×fasting glucose (mmol L−1)×22.5−1) Citation[9]. Current smoking was defined as daily smoking (yes/no).
In accordance with both national and international guidelines at the time, diagnosis of hypertension was based on three consecutive diastolic blood pressures ≥90 mmHg Citation[2]. Diagnosis of diabetes was confirmed by two fasting blood glucose values ≥6.7 mmol L−1, or by a two-hour glucose value of ≥11.1 mmol L−1 in an oral glucose tolerance test Citation[10]. However, the tolerance test was done only in selected cases.
From the Minnesota-coded electrocardiograms left ventricular hypertrophy was defined as (3.1 or 3.3 and 4.1–4.3) and/or (3.1 or 3.3 and 5.1–5.3) Citation[11].
End-points of the study included all fatal events of AMI. Information on these end-points from baseline to December 31, 2002 was ascertained by record linkage with the Swedish national mortality and the inpatient register. According to previous evaluation this method is considered valid Citation[12]. All variables studied in relation to mortality refer to the risk factor evaluation at the baseline examination.
Statistical analyses
The SPSS Base System for Macintosh 11.0 was used for data analyses. Proportions in baseline characteristics were age-standardized using the whole Skara population as standard in 10-year intervals. After controlling for proportionality, survival rates were examined by the Cox proportional hazard model with age as covariate, and expressed as hazard ratios (HR) with 95% confidence intervals (CI). The HRs for fasting blood glucose and cholesterol relate to the marginal effect on survival by 1 mmol L−1. The corresponding units are for HbA1c: 1%, for SBP: 10 mmHg, for DBP: 5 mmHg, for body mass index: 1 kg m−2, and for HDL cholesterol the HRs relate to one standard deviation (SD) difference. Fasting serum triglycerides and insulin resistance (HOMA) were dichotomized before analysis because of skewed distribution. Insulin resistance was dichotomized at the lower limit of the fourth quartile. Insulin-treated diabetic subjects (25 patients) were excluded from the analyses of insulin resistance (HOMA). Gender differences were examined by two-way interaction terms. Comparisons of continuous variables were analysed by general linear models with adjustment for differences in age.
Results
All together 894 patients were included in the study (377 men and 517 women). Of these, 204 (102 men and 102 women) had both hypertension and known type 2 diabetes, whilst 690 (275 men and 415 women) had hypertension only. The mean age of all men at inclusion was 66 years (± 10.5 years) and of all women 68 years (± 10.5 years).
During a mean follow-up of 8.7 years altogether 67 men (17.8%) and 66 women (12.8%) suffered from AMI. Fatal AMI occurred in 32 men (8.5%) and in 31 women (6.0%). The number of fatal AMIs compared with the total number of AMIs did not differ in the two sexes (47.7% in men and 47.0% in women). In patients with diabetes fatal AMI occurred in 15 men (14.7%) and in 16 women (15.7%), compared with 17 cases (6.2%) in men without diabetes and 15 cases (3.6%) in women without diabetes. High age was a predictor for AMI death in both sexes (HR by five years in men 1.4, CI 1.1–1.7; HR by five years in women 1.7, CI 1.3–2.1). Male gender predicted AMI mortality in the whole study population (HR 1.8, CI 1.1–2.9), a finding that was enforced in hypertensive patients without known diabetes (HR 2.7, CI 1.4–5.3), but disappeared in patients with both hypertension and known type 2 diabetes (HR 0.8, CI 0.4–1.7) (). The pattern of cardiovascular disease risk factors as predictors of AMI mortality was different in the two genders as shown in . Microalbuminuria was a strong predictor of AMI mortality in men, but not in women (). In patients without known cardiovascular disease systolic blood pressure significantly predicted AMI mortality in men (HR by 10 mmHg 1.0, CI 1.0–1.1), and diastolic blood pressure significantly predicted AMI mortality in women (HR by 5 mmHg 1.1, CI 1.0–1.1).
Figure 1. AMI survival curves for hypertensive men and women with and without known type 2 diabetes.
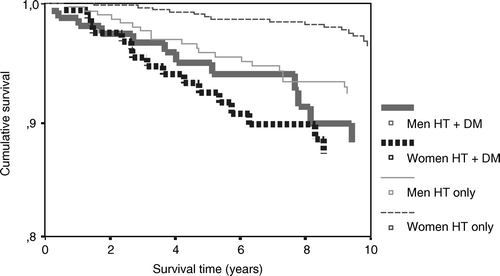
Figure 2. AMI survival curves for hypertensive men and women with and without known microalbuminuria.
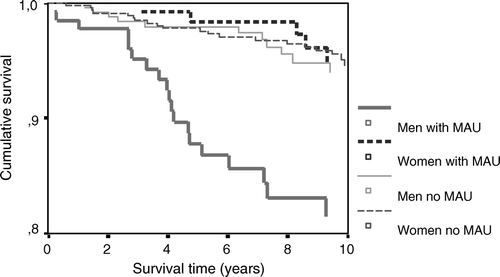
Table I. Baseline characteristics and corresponding hazard ratios for AMI mortality to December 31, 2002 in 894 subjects with hypertension in the Skaraborg Hypertension and Diabetes Project 1992–1993.
A two-way interaction term between gender (male gender versus female gender) and microalbuminuria significantly predicted AMI mortality (HR 4.2, CI 1.2–14.4). Correspondingly, a two-way interaction term between gender (female gender versus male gender) and known type 2 diabetes significantly predicted AMI mortality (HR 3.0, CI 1.1–8.4). This interaction remained significant when adjusted for age, smoking, serum cholesterol, BMI, and sedentary lifestyle (HR 3.6, CI 1.3–10.0).
Multivariate analyses included diastolic blood pressure, total cholesterol, triglycerides, known type 2 diabetes, previous cardiovascular disease, microalbuminuria, body mass index, and sedentary lifestyle. In men microalbuminuria (HR 3.4, CI 1.6–7.3) and previous cardiovascular disease (HR 2.2, CI 1.0–5.0) were significant predictors, whilst in women known type 2 diabetes (HR 4.0, CI 1.7–9.7), previous cardiovascular disease (HR 2.7, CI 1.1–6.5), and high fasting triglycerides (HR 2.6, CI 1.0–6.8) were significant predictors.
In the category of hypertensive patients without diabetes, men who suffered from AMI mortality had higher cholesterol (7.1 vs. 6.3 mmol L−1, p = 0.011) higher LDL cholesterol (4.6 vs. 4.1 p mmol L−1, p = 0.004) and higher prevalence of previous cardiovascular disease (47% vs. 18%, p = 0.010), left ventricular hypertrophy (24% vs. 4%, p = 0.001), and microalbuminuria (63% vs. 23%, p = 0.002), compared with hypertensive, non-diabetic men, who did not suffer from AMI mortality. In women no significant differences were found between similar patient categories.
Discussion
Principle findings
Cardiovascular disease risk factors remained predictors for AMI mortality in hypertensive patients, but with a different pattern in the two genders. Markers of organ damage are more important predictors in men, whereas markers of impaired glucose metabolism are more important predictors in women. Male gender is a strong predictor of AMI mortality in hypertensive patients without diabetes, but in hypertensive patients with known type 2 diabetes this relation disappears. These gender-specific patterns were emphasized by significant interaction terms and are supported by previous studies in the same population Citation[13], Citation[14], as well as in other populations Citation[15], Citation[16].
Strengths and weaknesses of the study
The strength of this study is the population-based design including practically all patients with known hypertension in the examined community. Additional strengths are the use of national and international criteria and standard procedures in ascertaining diagnoses Citation[2], as well as the previously developed and validated procedure of identifying AMI events Citation[13].
The greatest weakness of the study is the lack of information on changes in risk-factor levels and changes in antihypertensive treatment during the follow-up period. In addition, the number of patients treated with statins or anti-platelet therapy was not accounted for. However, these therapies were quite unusual in Swedish hypertensive patients during 1992–1993, considering that the 4S study Citation[17] was published in 1994 and the HOT study Citation[18] was published in 1998. Furthermore, coagulation factors that may have affected the outcome have not been investigated.
In the early 1990s the diagnoses of hypertension and diabetes were set at higher levels than today according to international standards Citation[2]. Some patients with diabetes according to modern standards were thus not diagnosed, but these patients should in general be earlier and milder cases of diabetes. Also, some patients with hypertension according to modern standards, as well as some cases of unidentified hypertensive patients, were not included in the study. Though these patients should have either a milder form or a shorter duration of hypertension, these circumstances imply that our study to some extent may have overestimated the risks. Furthermore, modern treatment goals for hypertension, diabetes and hyperlipidemia differ considerably from the risk-factor levels found at baseline in our study Citation[4–6], Citation[19]. However, similar risk-factor levels to those in our study have been demonstrated in several, also more recent, cross-sectional studies in primary care Citation[20–22]. Also, control of hypertension has been found to remain sub-optimal Citation[23] at similar levels to those previously demonstrated in the present study population Citation[24]. Consequently, the risk-factor control in our study could probably be considered to be representative of patients with hypertension in general.
In an earlier report we have demonstrated both diastolic and systolic blood pressure as predictors of all-cause mortality Citation[14]. In women systolic blood pressure was close to significance in relation to AMI mortality. The study population was quite old and, especially in men, a high proportion of cardiovascular disease was found. Both these factors are strong predictors of AMI mortality, and may thus have diluted the effect of blood pressure per se. It may also be that blood pressure levels have decreased secondary to cardiac disease in this elderly population. These theories are supported by the findings concerning blood pressure in patients without known cardiovascular disease.
Meaning of the study
Microalbuminuria was a strong and persistent predictor of AMI mortality in men, even in multivariate analyses, and seems to be more than a mirror image of high blood pressure and cardiovascular disease Citation[25]. Monitoring of microalbuminuria in hypertensive patients, therefore, is important.
The increased risk of AMI mortality in women with diabetes has previously been reported from the Skaraborg Project Citation[13]. In a recent Swedish cross-sectional survey female patients with diabetes had less favourable risk-factor control than corresponding male patients Citation[20], an association which was also found in a recent Finnish meta-analysis Citation[26]. However, this could not completely explain the higher mortality risk of diabetic female patients in our study, as this relation remained significant when adjusted for other risk factors. Moreover, it is possible that preventive and intervention procedures have not been applied to women to the same extent as to men Citation[20], Citation[27]. Another study largely explains the higher relative risk of AMI mortality in diabetic women by the persistently more favourable survival rate of women without diabetes Citation[28]. This is in accordance with the findings in our study. General practitioners should be observant regarding the high AMI risk in hypertensive women with diabetes and in hypertensive men with microalbuminuria.
The relatively high AMI mortality in hypertensive, non-diabetic men can be explained by clustering of cardiovascular disease risk factors in some of these patients. Such clustering of risk factors was not found in any subgroup of hypertensive, non-diabetic women, who therefore had a low AMI mortality. In an earlier study we demonstrated that patients with hypertension in combination with type 2 diabetes are characterized by clustering of risk factors Citation[2]. Consequently, patient categories with pronounced clustering of cardiovascular disease risk factors are associated with a high AMI mortality.
National and international guidelines emphasize the importance of treating all individual cardiovascular disease risk factors. The effectiveness of multi-factorial treatment has been proven in subjects with diabetes as well as in subjects with hypertension Citation[29], Citation[30]. Accordingly, methods to detect high-risk patients should be improved in the treatment of hypertensive patients. Screening programmes to detect and visualize risk-factor profiles of hypertensive patients should be feasible in primary care.
Unanswered questions
More research is warranted to explore differences in gender-specific mechanisms. In particular, the mechanism of risk-factor clustering in some hypertensive, non-diabetic men should be further investigated. Though we found different risk-factor patterns in men and women, these patterns cannot be taken as arguments to change clinical practice without further studies.
Conflicts of interest
None.
Acknowledgements
This study was supported by grants from the Swedish Medical Research Council, Skaraborg Institute, Skaraborg Primary Health Care, the Health and Medical Care Committee of the Regional Executive Board of the Region Västra Götaland, the NEPI Foundation (the Swedish Network for Pharmacoepidemiology), and the Faculty of Medicine, Lund University.
References
- Criqui MH, Barrett-Connor E, Holdbrook MJ, Austin M, Turner JD. Clustering of cardiovascular disease risk factors. Prev Med 1980; 9: 525–33
- Bøg-Hansen E, Lindblad U, Bengtsson K, Ranstam J, Melander A, Råstam L. Risk factor clustering in patients with hypertension and non-insulin-dependent diabetes mellitus. The Skaraborg Hypertension Project. J Int Med 1998; 243: 223–32
- Kannel WB. Status of risk factors and their consideration in antihypertensive therapy. Am J Cardiol 1987; 59: 80A–90A
- 1999 World Health Organization – International Society of Hypertension. Guidelines for the Management of Hypertension. J Hypertens 1999;17:151–83.
- 2003 European Society of Hypertension – European Society of Cardiology guidelines for the management of arterial hypertension. Guidelines Committee. J Hypertens 2003;21:1011–53.
- Swedish Council on Technology Assessment in Health Care. Måttligt förhöjt blodtryck. En systematisk litteraturöversikt [Moderately raised blood pressure. A systematic survey of publications]. SBU-rapport 170; 2004. Stockholm: SBU [SBU, Box 5650, 114 86 Stockholm, Sweden].
- Berglund G, Isacsson S-O, Rydén L. The Skaraborg Project – a controlled trial regarding the effect of structured hypertension care. Acta Med Scand 1979; 205[Suppl 626]: 64–8
- Rose GA. The diagnosis of ischemic heart pain and intermittent claudication in field surveys. Bull World Health Organ 1962; 27: 645–58
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–19
- World Health Organization Expert Committee. Diabetes mellitus. Technical Report Series no 742. GenevaSwitzerland: WHO; 1985.
- Rose GA, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular Survey Methods2nd edn. World Health Organization, Geneva 1982; 123–29
- Lindblad U, Råstam L, Ranstam J, Peterson M. Validity of register data on acute myocardial infarction and acute stroke: The Skaraborg Hypertension Project. Scand J Soc Med 1993; 1: 3–9
- Larsson CA, Gullberg B, Merlo J, Råstam L, Lindblad U. Female advantage in AMI mortality is reversed in patients with type 2 diabetes in the Skaraborg Project. Diabetes Care 2005; 28: 2246–8
- Bøg-Hansen E, Lindblad U, Gullberg B, Melander A, Råstam L. Survival in patients with hypertension treated in primary care: A population-based follow-up study in the Skaraborg Hypertension and Diabetes Project. Scand J Prim Health Care 2004; 22: 222–7
- Lee WL, Cape D, Cheung A, Zinman B. Impact of diabetes on coronary artery disease in women and men: A meta-analysis of prospective studies. Diabetes Care 2000; 23: 962–8
- Halimi JM, Forhan A, Balkau B, Novak M, Wilpart E, Tichet J, et al. DESIR Study Group. Is microalbuminuria an integrated risk marker for cardiovascular disease and insulin resistance in both men and women?. Cardiovasc Risk 2001; 8: 139–46
- The Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–9.
- Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet 1998; 351: 1755–62
- Alberti, KGMM, Zimmet, PZ, for the WHO Consultation. Definition, diagnosis and classification of diabetes mellitus and its complications, Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med 1998;15:539–53.
- Nilsson P, Theobald H, Journath G, Fritz T. Gender differences in risk factor control and treatment profile in diabetes: A study in 229 Swedish primary heath care centres. Scand J Prim Health Care 2004; 22: 27–31
- Kjellgren KI, Ahlner J, Dahlöf B, Gill H, Hedner T, Säljö R. Perceived symptoms amongst hypertensive patients in routine clinical practice – a population-based study. J Intern Med 1998; 244: 325–32
- Wolf-Maier K, Cooper RS, Kramer H, Banegas JR, Giampaoli S, Joffres MR, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension 2004; 43: 10–7
- Hedblad B, Nerbrand C, Ekesbo R, Johansson L, Midlöv P, Brunkstedt I, et al. High blood pressure despite treatment: Results from a cross-sectional primary healthcare-based study in southern Sweden. Scand J Prim Health Care 2006; 24: 224–30
- Bøg-Hansen E, Lindblad U, Gullberg B, Melander A, Råstam L. Metabolic disorders associated with uncontrolled hypertension: The Skaraborg Hypertension and Diabetes Project. Diabetes Obes Metab 2003; 5: 379–87
- Kvenild K, Romundstad S, Midthjell K, Kruger Ö, Hallan H, Holmen J. Microalbuminuria in treated hypertensives: Only a mirror image of cardiovascular risk?. Scand J Prim Health Care 2006; 24: 145–53
- Juutilainen A, Kortelainen S, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care 2004; 27: 2898–904
- Ferrara A, Williamson DF, Karter AJ, Thompson TJ, Kim X. Sex differences in quality of health care related to ischemic heart disease prevention in patients with diabetes. Diabetes Care 2004; 27: 2974–6
- Barret-Connor EL, Cohn BA, Wingard DL, Edelstein S. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernado Study. JAMA 1991; 265: 627–31
- Gaede P, Vedel P, Parving H-H, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: The Steno type 2 randomised study. Lancet 1999; 353: 617–22
- Agevall S, Fagerberg B, Berglund G, Schmidt C, Wendelhag I, Wikstrand J. for the Risk Factor Intervention Group, Sweden. Multiple risk intervention trial in high risk hypertensive men: comparison of ultrasound intima-media thickness and clinical outcome during 6 years follow-up. J Intern Med 2001; 249: 305–14