Abstract
Objective. The aim of this study was to estimate the rate of bacterial superinfection in patients with URTI by using on-site determination of C-reactive protein (CRP). Design. A prospective cohort study. Setting. A total of 30 primary care practices. Subjects. Patients with URTI. Intervention. The CRP value was determined at the first consultation and at a follow-up within 3–5 days. CRP values of 30 units (mg) or higher were considered to be an indication of bacterial involvement. Main outcome measures. CRP values during follow-up and duration of illness. Results. Among the 506 patients included, 73.1% exhibited a CRP value below the defined limit at their first visit and were considered to suffer from URTI of viral origin. The rate of subsequent bacterial superinfection was 8.1%. Compared with patients suffering from URTI of bacterial or viral origin the duration of illness in patients with bacterial superinfection was significantly longer. Conclusion. During follow-up of patients with URTI, the prevalence of bacterial superinfection detected by using a near patient CRP determination is surprisingly low. This result should help to reduce the prescription rate of antibiotics in primary care.
Infections of the upper respiratory tract (URTI) are among the most frequent reasons for encounter in general practice Citation[1–4]. The fear of prolonged sickness or of serious complications is known to result in an overuse of antibiotics although in the majority of cases the causal agent is of viral origin Citation[1], Citation[4], Citation[5]. The course of URTI of viral origin might be complicated by bacterial superinfection within the first days after onset of illness Citation[1]. Such patients benefit from specific treatment with antibiotics Citation[5], Citation[6].
However, precise detection of bacterial superinfection is difficult and its frequency is unknown; gold standards Citation[5], Citation[7], Citation[8] are not suitable for the primary care setting. In general, to differentiate between infections of viral or bacterial origin in clinical practice mainly erythrocyte sedimentation rate (ESR) and the determination of C-reactive protein (CRP) values have been used, since they increase in infections of bacterial origin Citation[9]. Therefore, the aim of this study was to observe the frequency of bacterial superinfection in patients with URTIs using on-site CRP determination at the onset of illness and for follow up within the primary care setting Citation[7], Citation[10–12].
Although the majority of infections of the upper respiratory tract (URTI) are caused by viruses, their course might be complicated by bacterial superinfection, the frequency of which is unknown.
The rate of bacterial superinfection in patients with URTI of viral origin is 8.1% as assessed by near patient CRP determination.
The duration of illness in these patients is significantly longer.
Material and methods
This prospective cohort study was conducted from November 2002 until March 2003 in the offices of 30 participating general practitioners (GPs). Each of the supporting GPs included up to 20 consecutive patients within the study period meeting the following inclusion criteria: an age over 4 years, elevated body temperature of more than 37.5° C, and at least one characteristic symptom of an URTI (sore throat, sinus pain, cough, stuffy nose) Citation[5], Citation[9], Citation[13].
Patients in whom pneumonia or another underlying disease associated with an elevated CRP value (e.g. pyelonephritis, rheumatoid arthritis, etc.) was known or suspected and persons already treated with antibiotics for a different reason were excluded Citation[9]. Written informed consent was obtained from all patients or their parents; the study was approved by the ethics committee of the Medical University of Vienna.
Study protocol and examinations
At the first consultation name, age, and a list of symptoms including the date of onset and the present health status was documented. Further, 25 µl capillary blood were taken from the patients and used to determine the CRP (CRP1) value as described Citation[14] using the whole blood NycoCard® CRP Single Test of Axis Shield Citation[10], Citation[11], Citation[15] and a portable colour densitometer (Nyocard Reader) to quantify the intensity of the membrane colour.
According to the test instructions and a number of earlier studies values of 30 units (mg/l) or higher were considered to be an indication of possible bacterial involvement, while values below 30 units were considered as a sign of probable viral infection Citation[7], Citation[9], Citation[12], Citation[16]. Patients with a CRP1 value higher than 30 units were supposed to receive conservative treatment Citation[6], Citation[17] such as antiphlogistics, antipyretics, inhalations, and other advice and were assigned to appropriate antibiotic prescription at their physician's option. Patients with a CRP1 value below 30 units were treated symptomatically only. All patients were asked to return to the office within 3–5 days after the first consultation for a recheck of their symptoms and for a second determination of CRP (CRP2). Only if the value had increased above 30 units bacterial superinfection was assumed and prescription of an antibiotic was recommended. In addition, all patients were asked to return or to call the office for notification of the first day of full recovery, which was taken as the end of the illness. In those cases where a sick leave certificate had been issued, the duration of absence from work was documented.
Analyses of the data
Based on the CRP determinations three groups of patients were defined: group 1 consists of patients with CRP1 values above the limit who were considered to have an infection of bacterial origin; patients in group 2 exhibited CRP values below the limit throughout the whole study period (“viral origin”); patients in group 3 had a low CRP1 value which increased within 3 to 5 days to CRP2 values above the limit (suspected “bacterial superinfection”).
Descriptive values (frequencies or mean values, respectively, and 95% confidence intervals, CI) are given and Pearson correlation coefficients (rho) were calculated. An analysis of variance was calculated in order to compare the mean values of the variable “duration of illness” of the three groups. The logarithm of the dependent variable was calculated in order to get a symmetrical distribution. Tukey's test for multiple comparisons was calculated.
A stepwise linear regression analysis was performed to analyse the influence on the duration of illness of: CRP, age, sex, cough, sore throat, sinus pain, rhinitis, fever, auscultatory findings, and antibiotic prescription. The probability to enter or remain in the model was set at 0.05, p-values and parameter estimates (p.e.) and R2 are reported. All analyses were carried out using SAS 8.02 (SAS Institute Inc., Cary, NC, USA) Citation[18].
Results
A total of 506 patients (261 female and 223 male) were recruited. Their mean age was 34.79 years (4–83 years, CI for the mean: 33.2–36.4); the majority of patients (88.15%) were ill for more than 3 days.
illustrates all participants and the different subgroups. Of 506 participants the following 90 patients were excluded from further analyses: 37 patients from group 2 (10%; CI 7.1–13.5) who received an antibiotic prescription despite their low CRP1 value and 36 patients (10.8%, CI 7.7–14.7) in which the CRP2 value could not be determined, as well as 7 patients from group 1 who did not receive antibiotic treatment despite their high CRP1 value and 10 patients who did not show up for the CRP2 determination.
Figure 1. Entire study population (first and second visit corresponding to CRP determinations). Hatched squares=: excluded patients.
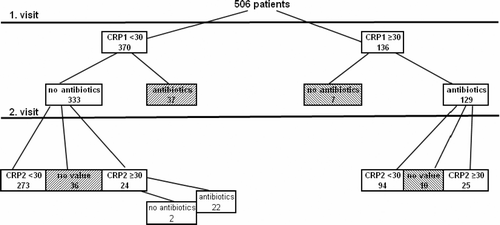
As can be seen in , about a quarter of the patients included showed a CRP1 value of 30 units or higher. Among remaining patients with a low CRP1 value (see ) 8.1% had an increase in their CRP2 value above the limit (). No gender difference for the distribution of CRP values could be found.
Table I. Distribution of CRP1 and CRP2 values above and below the limit at the first and second consultation.
The mean CRP values in the three study groups and their course throughout the study period were clearly different () and patients in group 3 showed the longest mean duration of illness (9.46 days). Analysis of variance resulted in a significant difference for the mean duration of illness between the three groups (p = 0.0002).
Table II. Mean CRP values and duration of illness referring to the three defined groups.
Tukey's test for multiple comparisons further shows a significant difference in the duration of illness for group 3 compared with group 1 (p = 0.01) as well as with group 2 (p = 0.0002). No significant difference can be found between patients in group 1 and those in group 2 (p = 0.12). By comparing patients in group 3 with those in group 2 a statistically significant difference can be found for their CRP1 values (p < 0.0001).
A high association between the two determinations of the CRP value could be found; Pearson's correlation coefficient is 0.35 (p < 0.0001; n = 456). Both CRP values correlate significantly with the duration of illness (CRP1: rho = 0.11, p = 0.02, n = 459; CRP2: rho = 0.29; p < 0.0001, n = 429).
Correlations between the length of illness and the age of the participant (rho = 0.21, p < 0.0001) as well as between the age and the CRP2 value (rho = 0.12, p = 0.01) could also be identified. A stepwise regression analysis showed that the age (p < 0.0001; p.e .=0.005), the logarithm of CRP1 values (p = 0.001; p.e.=0.05) and the presence of cough (p = 0.01; p.e.=0.14) all have a significantly positive influence on the duration of illness (R2=0.08).
Discussion
To our knowledge this is the first study to assess the rate of bacterial superinfection in patients with URTIs within the primary care setting.
Among the patients included, 73.1% exhibited a CRP1 value below the limit at their first visit. Although specific identification of the causal agent in the different secretions – considered to be the gold standard in the secondary care setting – was not performed, it can be assumed that CRP values below 30 units in patients with URTIs are caused by viral infections Citation[11].
In contrast to the high rate of antibiotics prescribed for URTI Citation[4], Citation[19–21], only approximately one-quarter of URTIs in our study appear to be of bacterial origin (see ). These results compare well with those of Gulich Citation[7] who studied the diagnostic accuracy of near patient CRP measurement in patients with pharyngitis.
Similarly, an increase in the CRP value at the second determination can be considered to indicate bacterial superinfection. Assessed by CRP determination, the rate of bacterial superinfection among patients with URTI of viral origin is 8.1% (see ). From a previous study, the frequency for bacterial superinfection based on 41 patients can be calculated to be 4.88% Citation[9]. However, physicians tend to prescribe antibiotics rather quickly Citation[20–22], because in many countries there is no reliable near patient test for the causal agent routinely available Citation[10], Citation[23] and the rate of bacterial superinfection is therefore overestimated. In addition, patients with URTI in primary care might demand antibiotic medication Citation[17], Citation[20].
As can be seen in , patients in group 2 with low CRP1 values also show low CRP2 values, while patients in group 3 showed initially higher CRP1 values than those in group 2. This might indicate the early stage of an initial bacterial infection in group 3. Supporting the fundamental difference between the two groups the duration of illness in patients in group 3 is clearly longer than that of patients in group 2 or group 1.
The strength of our study is that it has been performed in the primary care setting where the near patient CRP determination helps to improve clinical decision-making in patients with suspected bacterial superinfection. Based on the feedback of participating GPs, both patients and physicians appreciate the quick test result as a rationale for subsequent shared decisions about the treatment Citation[10], Citation[23]. In addition, this procedure Citation[15], Citation[24], Citation[25] is of minimal discomfort for patients when compared with intravenous blood sampling at an outpatient laboratory.
Some limitations of our study have to be acknowledged. First, a total of 90 patients of the initial 506 patients included dropped out (). This might influence the results of our study. Second, the parameter followed (CRP) and its cut-off level used can be questioned. However, the determination of CRP is used in a variety of conditions to differentiate between bacterial and viral infections Citation[26], Citation[27]. Further, the cut-off level chosen refers to the test instructions and to a number of earlier studies Citation[9], Citation[14], Citation[16], Citation[27]; in particular, those dealing with URTIs have shown that CRP values in patients with viral infections mostly remain below 30 units Citation[9], Citation[12], Citation[16]. Despite this we might have included patients with a CRP1 value above the limit as bacterially infected, while their CRP values could have been increased due to other unrecognised reasons. Overall, this would have resulted in an overestimation of bacterial infection and superinfection, respectively. We tried to address this possibility by strict exclusion criteria based on the GP's knowledge of the patient's history. Third, our study has been performed within a certain time period in Central Europe during the winter season. Therefore, we cannot exclude that the relationship between viral and bacterial infections might be different during other time periods or in other regions Citation[1], Citation[28].
In summary, our study shows that bacterial superinfections during follow-up of URTIs can be estimated easily and that their frequency is surprisingly low (8.1%); the duration of illness in these patients is significantly longer. These observations and conclusions might help to reduce unnecessary antibiotic prescriptions for URTIs Citation[13], Citation[20], Citation[22], Citation[23], Citation[29] and to slow down the increase of antibiotic resistance Citation[24], Citation[30].
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Role of the funding source
The CRP test kits and densitometers for this study were kindly provided by Technoclone® and Axis Shield. The companies had no influence on the design of the study or on data collection, analyses and interpretation. No other funding was provided.
Acknowledgements
The authors would like to thank the following participants of the Austrian Research Network of General Practitioners who contributed patient data to this study: R. Aumayr, H. Bachler, G. Bartke, H. Berger, F. Burghuber, C. Dachs, A. Fragner, B. Fürthauer, R. Glehr, W. Heidinger, W. Hockl, T. Horvatits, E. Jenull, E. Kepplinger, P. Kufner, E. Maier, M. Mayerhofer, B. Mirtl, E. Moser-Rapf, B. Panhofer, P. Pichler, A. S. Puri-Jobi, H. Racic, H. Radakovits, H. Ragossnig, E. Rebhandl, J. Schützenberger, A. Seiwald, K. Wolfram, W. Zillig.
The following colleagues participated in the pilot study: C. Adensamer, E. Maier, C. Sellner, W. Spiegel, H. Toenies.
After a pilot study including 100 patients (presented at the WONCA world Conference in Durban 2001) some adjustments to the earlier study protocol and to the datasheet were made.
References
- Kirkpatrick GL. The common cold. Prim Care 1996; 23: 657–75
- Andre M, Eriksson M, Molstad S, Stalsbylundborg C, Jacobsson A, Odenholt I. The management of infections in children in general practice in Sweden: A repeated 1-week diagnosis-prescribing study in 5 counties in 2000 and 2002. Scand J Infect Dis 2005; 37: 863–9
- Hing E, Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2004 summary. Adv Data 2006; 374: 1–33
- Reveiz L, Cardona AF, Ospina EG. Antibiotics for acute laryngitis in adults. Cochrane Database Syst Rev 2005(1): CD004783.
- Arroll B, Kenealy T. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev 2005(3): CD000247.
- Thomas M, Del Mar C, Glasziou P. How effective are treatments other than antibiotics for acute sore throat?. Br J Gen Pract 2000; 50: 817–20
- Gulich MS, Matschiner A, Gluck R, Zeitler HP. Improving diagnostic accuracy of bacterial pharyngitis by near patient measurement of C-reactive protein (CRP). Br J Gen Pract 1999; 49: 119–21
- Marple BF, Brunton S, Ferguson BJ. Acute bacterial rhinosinusitis: A review of US treatment guidelines. Otolaryngol Head Neck Surg 2006; 135: 341–8
- Melbye H, Hvidsten D, Holm A, Nordbo SA, Brox J. The course of C-reactive protein response in untreated upper respiratory tract infection. Br J Gen Pract 2004; 54: 653–8
- Cohen R, Romain O, Levy C, et al. [Impact of CRP rapid test in management of febrile children in paediatric emergency units of Ile-de-France]. Arch Pediatr 2006; 13: 1566–71
- Hobbs FD, Kenkre JE, Carter YH, Thorpe GH, Holder RL. Reliability and feasibility of a near patient test for C-reactive protein in primary care. Br J Gen Pract 1996; 46: 395–400
- Hjortdahl P, Landaas S, Urdal P, Steinbakk M, Fuglerud P, Nygaard B. C-reactive protein: A new rapid assay for managing infectious disease in primary health care. Scand J Prim Health Care 1991; 9: 3–10
- File TM, Jr, Hadley JA. Rational use of antibiotics to treat respiratory tract infections. Am J Manag Care 2002; 8: 713–27
- Dahler-Eriksen BS, Lassen JF, Petersen PH, Lund ED, Lauritzen T, Brandslund I. Evaluation of a near-patient test for C-reactive protein used in daily routine in primary healthcare by use of difference plots. Clin Chem 1997; 43: 2064–75
- Esposito S, Tremolati E, Begliatti E, Bosis S, Gualtieri L, Principi N. Evaluation of a rapid bedside test for the quantitative determination of C-reactive protein. Clin Chem Lab Med 2005; 43: 438–40
- Lehtinen P, Jartti T, Virkki R, et al. Bacterial coinfections in children with viral wheezing. Eur J Clin Microbiol Infect Dis 2006; 25: 463–9
- Fischer T, Fischer S, Kochen MM, Hummers-Pradier E. Influence of patient symptoms and physical findings on general practitioners’ treatment of respiratory tract infections: a direct observation study. BMC Fam Pract 2005; 6: 6
- SAS Institute Inc SAS/STAT. User's Guide Version 8. Cary, NC: SAS Inc; 1999.
- Steinman MA, Landefeld CS, Gonzales R. Predictors of broad-spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA 2003; 289: 719–25
- Rutschmann OT, Domino ME. Antibiotics for upper respiratory tract infections in ambulatory practice in the United States, 1997–1999: Does physician specialty matter?. J Am Board Fam Pract 2004; 17: 196–200
- Roumie CL, Halasa NB, Grijalva CG, et al. Trends in antibiotic prescribing for adults in the United States––1995 to 2002. J Gen Intern Med 2005; 20: 697–702
- Butler CC, Rollnick S, Pill R, Maggs-Rapport F, Stott N. Understanding the culture of prescribing: Qualitative study of general practitioners’ and patients’ perceptions of antibiotics for sore throats. BMJ 1998; 317: 637–42
- Bjerrum L, Gahrn-Hansen B, Munck AP. C-reactive protein measurement in general practice may lead to lower antibiotic prescribing for sinusitis. Br J Gen Pract 2004; 54: 659–62
- Papaevangelou V, Papassotiriou I, Sakou I, et al. Evaluation of a quick test for C-reactive protein in a pediatric emergency department. Scand J Clin Lab Invest 2006; 66: 717–21
- Evrard B, Roszyk L, Fattal S, Dastugue B, Sapin V. Evaluation of rapid, semi-quantitative assay of C-reactive protein in whole blood, Actim CRP]. Ann Biol Clin 2005; 63: 525–9
- Marcus N, Mor M, Amir L, Mimouni M, Waisman Y. The quick-read C-reactive protein test for the prediction of bacterial gastroenteritis in the pediatric emergency department. Pediatr Emerg Care 2007; 23: 634–7
- Sormunen P, Kallio MJ, Kilpi T, Peltola H. C-reactive protein is useful in distinguishing Gram stain-negative bacterial meningitis from viral meningitis in children. J Pediatr 1999; 134: 725–9
- Van der Velden K, Paget J, Meijer A, Brown C, Meerhoff T, Faassen A. Annual Report 2004–2005 influenza season. Utrechtthe Netherlands. NIVEL, 2006. http://www.eiss.org/html/annual_reports.html.
- Hadley JA, Pfaller MA. Oral beta-lactams in the treatment of acute bacterial rhinosinusitis. Diagn Microbiol Infect Dis 2007; 57: 47S–54S
- Nasrin D, Collignon PJ, Roberts L, Wilson EJ, Pilotto LS, Douglas RM. Effect of beta lactam antibiotic use in children on pneumococcal resistance to penicillin: prospective cohort study. BMJ 2002; 324: 28–30