Abstract
Objective. Investigation of the clinical course of infection with Giarda lamblia after a large outbreak in an area where Giardia is not endemic in humans. Design. A cohort of patients from primary healthcare with clinically defined giardiasis was investigated by retrospectively analysing data from the patients’ medical records. Setting. Urban primary healthcare setting in Bergen, Norway. Subjects. From a population (n = 7100) assigned to two general practice clinics located in the outbreak area 134 patients met the inclusion criteria of at least one of the following: typical symptoms for at least one week, detection of Giarda lamblia in stool samples, or receiving a specific diagnosis. Of these, 119 gave consent to take part in the study. Main outcome measures. Proportion of patients with clinical giardiasis identified by detection of parasites in stool samples. Proportion of patients with prolonged disease and recurring symptoms. Results. A positive test for Giardia lamblia was found in 55% (66/119) of the patients. Specific treatment was given to 89 patients, and after treatment 36% (32/89) returned to their doctor because they experienced recurring symptoms. Compared with those not returning a significantly higher proportion of this group had seen their GP for other GI complaints in the previous two years. Conclusion. Laboratory-based diagnosis missed a substantial number of patients falling sick with giardiasis during the outbreak. One-third of the patients experienced recurring symptoms after treatment, and there was an association between previous gastrointestinal complaints and recurrence of symptoms.
In the autumn of 2004 a widespread community-based outbreak of giardiasis occurred in Bergen, the second largest city in Norway. There were approximately 1300 laboratory-confirmed cases and an estimated 2500 patients treated for giardiasis. The source of infection was a water reservoir supplying water to 42 000 of the city's 250 000 inhabitants Citation[1]. In North America and several European countries Giardia lamblia is the most commonly identified pathogen causing waterborne illness. In Norway, this was the first time a large giardiasis outbreak had been recognized.
Several outbreaks in other countries have been described focusing on epidemiologic aspects estimating the size of the outbreaks, investigating paths of transmission, and discussing implications for the community Citation[2–4]. The clinical course of giardiasis during outbreaks has been subject to relatively little attention, and our knowledge is based on smaller studies Citation[5–7]. In this study we defined a cohort of patients with giardiasis by clinical criteria. We describe the course of giardiasis in this cohort according to the patients’ medical records, with special attention to prolonged disease and recurring symptoms.
Material and methods
Norway has a registered patient list system for general practice. Patients are supposed to visit their general practitioner (GP) for all first-time contacts with the health services, regarding both acute and chronic illness. In addition, there are emergency wards open to the public outside regular opening hours.
Giardia lamblia is a common cause of waterborne illness. We report on the course of a large outbreak in Norway as registered in general practice.
During an outbreak stool samples positive for Giardia lamblia will identify a limited proportion of affected patients.
A high proportion of patients with giardiasis re-visited their doctors with recurrence of symptoms after treatment.
A history of previous gastrointestinal complaints was associated with higher risk of recurring symptoms.
The participants in this study were recruited from two general practice clinics located in the area supplied with water from the contaminated reservoir. Eight GPs, among them two of the authors (KAW and GR), work at these clinics, serving a total of 7100 individuals.
We aimed to include all patients who had been in contact with these clinics due to giardiasis in the period 1 August 2004 to 28 February 2005. This time-frame was based on a report from the local health authorities stating that the first patients experienced symptoms of giardiasis at the beginning of September 2004 Citation[8].
We conducted an electronic search of the medical records (Infodoc® and Winmed®) including diagnoses in ICPC-2 that could imply giardiasis: D01 ‘abdominal pain/cramps general’, D02 ‘abdominal pain epigastric’, D06 ‘abdominal pain localized other’, D07 ‘dyspepsia/indigestion’, D08 ‘flatulence/gas/belching’, D09 ‘nausea’, D11 ‘diarrhoea’, D18 ‘change in faeces/bowel movements’, D70 ‘gastrointestinal infection’, D73 ‘gastroenteritis presumed infection’, D87 ‘stomach function disorder’, and D96 ‘worms/other parasites’. Our choice of diagnoses was checked against the opinions of the other doctors at the clinics. Additionally, we did a manual search of all appointment books for the period 1 August to 15 November to identify consultations where the stated reason suggested infection with Giardia lamblia. We then read through the medical records for all patients identified to see whether they met the inclusion criteria set to define the diagnosis of giardiasis in this study ().
Table I. Inclusion criteria for the clinical diagnosis of giardiasis.
All data describing the clinical features and course of the disease were registered. The chronology of events was recorded by week number. We also made a note of any gastrointestinal symptoms or illness described in the medical record prior to the period of inclusion.
Both centres submitted stool samples to the laboratory at Haukeland University Hospital, Bergen, where testing for parasites was performed by direct microscopy or a commercial antigen-detecting test Citation[9], Citation[10].
All data were registered and analysed in SPSS version 14.0. Chi-squared test or Fisher's exact test was used to test differences between proportions, and Student's t-test to test differences for continuous variables. The level of statistical significance was set at p < ;0.05.
Results
We identified 134 patients who met the inclusion criteria, and they were asked to consent to take part in the study. Nine patients declined, and six patients did not reply. Consequently, 119 patients were included. Several patients met more than one of the inclusion criteria, 103 were included based on the symptoms described, 66 had a positive faecal test for Giardia lamblia, and 69 were given the specific diagnosis “giardiasis” by the consulting doctor.
In the study population there was a significantly higher proportion of females and young adults compared with the total patient population at the two clinics (). During the last two years prior to the outbreak 15 of the 119 patients included (13%) had seen their GP about GI complaints.
Table II. Age and gender distribution of the total patient population and the giardia patient population at the two general practices.
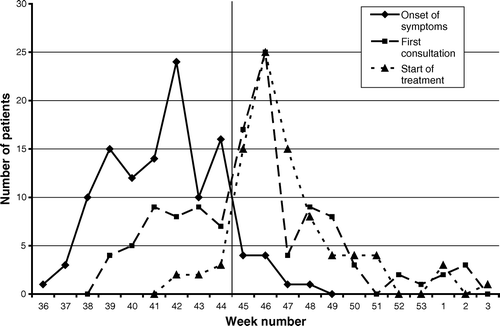
The first patient fell ill in the last week of September 2004. During the following weeks an increasing number of patients consulted their GPs with symptoms of giardiasis, culminating after the outbreak became publically known on 2 November 2004 (week 45) (). During the study period the outbreak generated a total of 216 visits to the GP's office, and another 189 telephone calls or letters. Thus, these patients were in contact with the doctor 3.4 times on average. In the acute phase of the disease six patients (5%) were admitted to hospital for emergency care because of severe dehydration.
Figure 1. Development of outbreak week by week autumn 2004 and winter 2005. Note: Outbreak recognized beginning of week 45 as indicated by vertical line.
Stool samples from 107 patients (90%) were analysed for Giardia lamblia. In 95% of these cases only one sample for each patient was analysed. A positive test was found in 55% (66/119) of the population, and the mean age was lower in this group ().
Table III. Characteristics of patients with and without positive stool sample during an outbreak of giardiasis.
Treatment was given to 75% (89/119) of the patients, and they all received metronidazole, the only drug registered in Norway for the treatment of giardiasis. Several drug regimens were chosen, lasting three, seven or 10 days, and the total dose ranged from four to 12 grams. The data were analysed by duration and dose, and stratified into “high dosage” and “low dosage”, but there was no trend or significant difference in success rate between drug regimens (data not shown). After treatment 36% (32/89) of the treated patients saw their GP due to recurring symptoms. The interval between treatment and recurrence of symptoms varied between 0 and 60 days (median seven days). In the group experiencing recurrence of symptoms, a significantly higher proportion had seen their GP for some kind of GI complaint during the last two years prior to the outbreak (). We found no other variables that could explain the association. For instance, when comparing the rate of Giardia-positive stool samples between the groups with or without previous GI-complaints there was no significant difference either before treatment (60% (9/15) vs. 55% (57/104), p = 0.79) or after recurrence of symptoms (50% (4/8) vs. 53% (9/17), p = 1.00).
Table IV. Characteristics of patients with recurring symptoms after treatment for giardiasis.
A second prescription for metronidazole was given to 28% (25/89) of the patients, and most often both the daily dose and the duration were increased. After the second treatment 16% (14/89) returned once more, and 11% (10/89) were treated a third time. Five patients (6%) who did not get well after the third treatment were referred to the hospital's outpatient clinic for further investigations and follow-up.
Discussion
In this study from primary healthcare we investigated an acute outbreak of giardiasis in a population previously unexposed to Giardia lamblia. Nearly one half of the patients who met validated clinical criteria for giardiasis were not identified by detection of Giardia lamblia in stool samples. A large proportion of the patients experienced recurring symptoms, and the risk was correlated to whether the patients had seen their GP for gastrointestinal complaints during the last two years prior to their infection.
The outbreak in Bergen is well defined with established time frame and source of infection Citation[1]. This allows the possibility to observe the course of the disease less influenced by other factors. We have gathered information from an extended period of time following the outbreak as it was evident to the medical centres. The amount and accuracy of the data entered might differ between GPs, and change with time. As the GPs grew more familiar with the symptoms it is likely that the amount of information decreased. This puts certain limitations on what can be extracted from the data; hence, we made no attempt to score severity of symptoms.
Some patients may have been lost to our study because they only attended the city's central emergency ward, rather than seeing their GP. This number should be small as most patients in the overall outbreak were diagnosed by their GPs. Only 15% of positive stool samples were obtained from the central emergency ward Citation[11] and a substantial proportion of these patients were probably university students who do not have their assigned GP in Bergen.
Non-participants had a slightly higher mean age compared with participants (45 vs. 37 years, p = 0.107). They did not differ from the study population when compared by gender, faecal analysis, or treatment. We therefore conclude that the internal validity is high. The demographic distribution with a predominance of young adults and females is also seen in the overall outbreak Citation[1], and we find little reason to suspect selection bias.
It is difficult to assess whether our findings are applicable to populations where giardiasis is endemic. The clinical and epidemiological features of the disease might be different in a previously unexposed population. For instance, we found few children in our study, whereas globally giardiasis is more prevalent in children Citation[7].
Any test (clinical or laboratory) used to diagnose giardiasis would have a high positive predictive value during the outbreak due to high prevalence of the infection. A case definition of giardiasis based solely on positive stool samples would have identified only 55% of the patients in this study. We chose a clinical case definition Citation[12] because this would identify a truer patient population. Relying on positive stool samples alone would exclude patients where the consulting doctor did not ask for samples, which was likely before recognition of the outbreak. Also, the local health authorities at one time advocated that no stool samples should be taken if clinical features made the diagnosis seem certain. Sensitivity of the tests was also reduced since only one sample was submitted in most cases. Possible reasons why a recommended three samples were not investigated could be that the symptoms were typical, that the laboratory result would not affect choice of treatment or that patients were reluctant to deliver the samples. Sensitivity could also be influenced by the long period from onset of symptoms until the samples were submitted, five weeks on average. Our data also suggest that sensitivity might be influenced by the age of the patients.
There was a delay in detection of the outbreak Citation[1] and accordingly delay in diagnosis and treatment for some patients. Our data do not show any long-term consequences of delayed treatment.
Although the number of patients is small, we found a statistically significant association between previous gastrointestinal complaints and recurrence of symptoms after treatment. One could argue that patients with prior complaints may have consulted their GP because of chronic symptoms and did not really have giardiasis, but there was no difference in rate of positive stool samples before treatment in the two groups. A previous study indicated that in giardiasis patients retrospectively diagnosed with previous irritable bowel syndrome (IBS) the infection can elicit IBS symptoms, and that it is the symptoms of IBS and not giardiasis that prevail Citation[13]. These results are hard to evaluate since the eradication rate of the parasite was low (36%). Another study from the Bergen outbreak has shown that some patients suffered prolonged bowel symptoms after eradication of parasites up to 18 months after the initial infection Citation[14]. It is unclear whether these patients had symptoms prior to their infection, and they are most often given the diagnosis post-infectious IBS. Several microorganisms have been shown to cause this condition Citation[15], but the mechanisms are not clear. Our study indicates that there may be some connection between a kind of fragility in the gut and the reaction to Giardia lamblia. Further research is needed to highlight this issue.
Ethical approval
This study has been approved by the Regional Committee for Medical Research Ethics, and by the Ombudsman for Privacy in Research, Norwegian Social Science Data Services.
Conflict of interests: None.
Acknowledgements
The authors would like to thank their fellow GPs at Fjellsiden legesenter and Kalfaret legesenter for encouragement and allowing them access to the medical records of their patients.
This study was partly funded by a grant from the Norwegian Medical Association.
References
- Nygard K, Schimmer B, Sobstad O, Walde A, Tveit I, Langeland N, et al. A large community outbreak of waterborne giardiasis: Delayed detection in a non-endemic urban area. BMC Public Health 2006; 6: 141
- Lopez CE, Dykes AC, Juranek DD, Sinclair SP, Conn JM, Christie RW, et al. Waterborne giardiasis: A communitywide outbreak of disease and a high rate of asymptomatic infection. Am J Epidemiol 1980; 112: 495–507
- Neringer R, Andersson Y, Eitrem R. A water-borne outbreak of giardiasis in Sweden. Scand J Infect Dis 1987; 19: 85–90
- Shaw PK, Brodsky RE, Lyman DO, Wood BT, Hibler CP, Healy GR, et al. A communitywide outbreak of giardiasis with evidence of transmission by a municipal water supply. Ann Intern Med 1977; 87: 426–32
- Walzer PD, Wolfe MS, Schultz MG. Giardiasis in travelers. J Infect Dis 1971; 124: 235–7
- Wright RA, Spencer HC, Brodsky RE, Vernon TM. Giardiasis in Colorado: An epidemiologic study. Am J Epidemiol 1977; 105: 330–6
- Farthing MJ. Giardiasis. Gastroenterol Clin North Am 1996; 25: 493–515
- Tveit I, Sobstad O, Kalland I, Seim A, Arnesen R, Fennell P. Giardia-utbruddet i Bergen høsten 2004 [The Giardia outbreak in Bergen autumn 2004]. Report from the local health authorities. Bergen; 2005.
- Garcia LS, Shimizu RY, Novak S, Carroll M, Chan F. Commercial assay for detection of Giardia lamblia and Cryptosporidium parvum antigens in human fecal specimens by rapid solid-phase qualitative immunochromatography. J Clin Microbiol 2003; 41: 209–12
- Johnston SP, Ballard MM, Beach MJ, Causer L, Wilkins PP. Evaluation of three commercial assays for detection of Giardia and Cryptosporidium organisms in fecal specimens. J Clin Microbiol 2003; 41: 623–6
- Steen K, Damsgaard E. Giardiaepidemien i og Bergen legevakt [The Giardia epidemic in 2004 and out-of-hours service in Bergen]. Tidsskr Nor Laegeforen 2007; 127: 187–9
- Hopkins RS, Juranek DD. Acute giardiasis: An improved clinical case definition for epidemiologic studies. Am J Epidemiol 1991; 133: 402–7
- D'Anchino M, Orlando D, De Feudis L. Giardia lamblia infections become clinically evident by eliciting symptoms of irritable bowel syndrome. J Infect 2002; 45: 169–72
- Hanevik K, Hausken T, Morken MH, Strand EA, Morch K, Coll P, et al. Persisting symptoms and duodenal inflammation related to Giardia duodenalis infection. J Infect 2007; 55: 524–30
- Spiller R, Campbell E. Post-infectious irritable bowel syndrome. Curr Opin Gastroenterol 2006; 22: 13–17