Abstract
Objective. Searching for useful diagnostic tools to discriminate between asymptomatic bacteriuria (ASB) and acute cystitis, this study compared urinary levels of cytokines/chemokines and leukocyte esterase in three groups of elderly subjects; those with acute cystitis, those with ASB, and those without bacteriuria. Design. Comparative laboratory. Setting. Primary care. Subjects. A total of 16 patients with acute cystitis, 24 subjects with ASB, and 20 controls without bacteriuria, all of whom were aged 80 or over. Main outcome measures. Urinary levels of IL-1β, TNF-α, IL-12, IL-18, CXCL1 (GRO-α), CXCL8 (IL-8), CCL2 (MCP-1), IL-6, IL-10, and leukocyte esterase. Results. Urinary levels of CXCL1, CXCL8, and IL-6 were significantly higher in acute cystitis patients than in the ASB group. The sensitivities and specificities for CXCL8, IL-6, and leukocyte esterase to discriminate between acute cystitis and ASB were 63% (95% CI 36–84) and 96% (95% CI 77–100) (cut-off > 285 pg/mg creatinine), 81% (95% CI 54–95) and 96% (95% CI 77–100) (cut-off > 30 pg/mg creatinine), and 88% (95% CI 60–98) and 79% (95% CI 57–92) (cut-off > 2, on a scale of 0–4), respectively. Conclusions. The results indicate that measurement of urinary cytokines, and also leukocyte esterase, when using a cut-off value > 2, could be useful in clinical practice to discriminate between symptomatic and asymptomatic urinary tract infections in the elderly. A combination of IL-6 and leukocyte esterase could be even more useful. This needs to be evaluated in prospective studies on the diagnosis and treatment of urinary tract infections in an elderly population.
Urinary tract infections (UTIs), and predominantly acute cystitis, are common in the elderly. In this age group in Sweden, they account for nearly half of the antibiotic prescriptions Citation[1]. Asymptomatic bacteriuria (ASB) is also common in the elderly, 19% in women and 9% in men in a Swedish community-living population aged ≥ 80 years Citation[2]. In institutional living the prevalence of ASB in women is 30–50% Citation3–5.
There is convincing evidence that, except in pregnant women, ASB is a harmless condition and should not be treated Citation[6]. However, it is often difficult to differentiate between acute cystitis and ASB in daily practice Citation[7]. This may be due to patients’ difficulties in communicating their symptoms because of cognitive impairments or when the elderly patient presents diffuse systemic symptoms without apparent focal urinary signs. Then a finding of bacteriuria may be difficult to interpret, and may lead to a failure to detect the true causes of the symptoms as well as to overuse of antibiotics.
In a UTI, both immune cells and various other cell types such as endothelial and uro-epithelial cells participate in the host response. Cytokines play an important role in the regulation of the local inflammatory response to infections Citation8–19.
It is often difficult to discriminate between acute cystitis and asymptomatic bacteriuria (ASB) in the cognitively impaired, or when elderly patients present diffuse symptoms.
A urinary dipstick test for leukocyte esterase may be helpful in separating acute cystitis from ASB.
Testing for urinary IL-6 may improve diagnosis, especially when combined with testing for urinary leukocyte esterase.
More accurate diagnosing may contribute to a reduction of antibiotic overuse.
Although ASB is asymptomatic by definition, it is not merely a colonization of bacteria in the urinary tract. ASB can be regarded as an asymptomatic infection, since ASB is associated with leukocyturia, elevated urinary cytokines, and an elevated urinary antibody to uropathogens Citation[20], Citation[21]. However, the levels of cytokines/chemokines in the urine of elderly people with regard to ASB and acute cystitis have not yet been fully investigated.
The aim of this exploratory study was to investigate the levels of cytokines/chemokines and leukocyte esterase in the urine of elderly patients with acute cystitis as compared with individuals with ASB and without bacteriuria, searching for possible diagnostic tools to discriminate between ASB and acute cystitis.
Material and methods
Patients with acute cystitis
Sixteen consecutive patients ≥ 80 years old (15 women and one man, median age 85 years), diagnosed with an acute cystitis at Britsarvets primary healthcare centre in Falun, Sweden, between March and September 2005, were included. The diagnosis required new onset of dysuria, frequency, and/or urgency and significant bacterial growth (in two subjects the urine was not cultured but tested positive for nitrite).
Subjects with ASB
Twenty-four individuals with ASB (22 women and two men, median age 86 years) were recruited from a population-based study of ASB in the community-dwelling elderly Citation[2]. They all had growth of ≥ 108 CFU/l of Escherichia coli in two consecutive urine samples, obtained within 1–2 weeks, without new onset of dysuria, frequency, and/or urgency.
Subjects without bacteriuria
Twenty controls (15 women and five men, median age 84 years) with one negative urinary culture and no symptoms of UTI were recruited from the same population-based study mentioned above Citation[2].
Urine samples
Urine cytokine detection
All urine samples were stored at −20°C until tested. After thawing, they were centrifuged at 3000 rpm for 10 min at 4°C before analyses. Quantitative determination of IL-1β, TNF-α, IL-12, CXCL8, IL-6, and IL-10 urine concentrations were performed using a human Cytometric Bead Array Inflammation kit (CBA; Becton Dickinson Biosciences, Stockholm, Sweden), analysed by flow cytometry (FACS Canto, Becton Dickinson), according to the manufacturer's instructions. The levels of CXCL1 and CCL2 were analysed using human Quantikine® ELISA (R&D Systems, Abingdon, UK). CCL2 was analysed according to the manufacturer's instructions. There was no protocol for urine samples for CXCL1, and therefore spike, recovery, and linearity experiments were performed to validate the assays, as recommended by the manufacturer.
Urine creatinine detection
Urine creatinine concentration was measured using a modified Jaffe reaction (ADVIA 1650, Bayer, Stockholm, Sweden), and the ratios of cytokine/creatinine (pg/mg) were calculated to normalize sample results for dilution.
Urine leukocyte esterase detection
Urinary leukocyte esterase was analysed semi-quantitatively using Clinitek 50 (Bayer) on a urinary dipstick (Multistix® 5, Bayer), showing the leukocyte esterase concentration on a scale from 0 to 4.
Statistics
The software used was the SPSS package for Windows, Rel. 14.0.0, 2005. Urine cytokine concentrations in the acute cystitis and ASB groups and negative controls were compared using the Mann–Whitney U-test. P-values < 0.05 were considered significant. Yates's correction was used when calculating confidence intervals for sensitivity and specificity.
Results
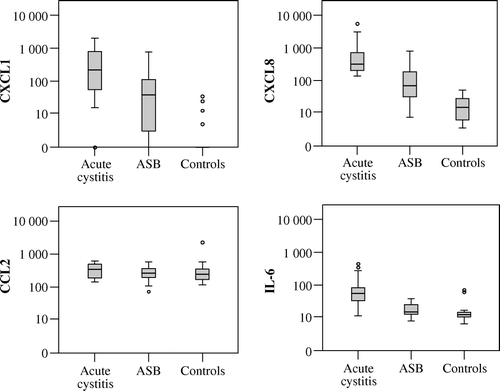
Urinary levels of tested cytokines and chemokines, corrected for urine creatinine, are presented in , where the levels in patients with acute cystitis, ASB, and negative controls are compared. We found significantly higher levels of urine CXCL1, CXCL8, and IL-6 in subjects with ASB than in negative controls, and even higher levels in acute cystitis.
Table I. Urinary levels of tested cytokines in patients with acute cystitis, ASB, and negative controls.
Comparisons without correction for urine creatinine revealed similar significant differences among the three groups regarding CXCL1 and CXCL8. For IL-6 there were higher urine levels in acute cystitis than in ASB, while there was no difference when the ASB group was compared with negative controls.
shows the distribution of CXCL1, CXCL8, CCL2, and IL-6 in the three different groups, visualizing the interquartile range and median values. In CXCL1 there were non-detectable urinary levels in most negative controls and occasionally in the ASB and acute cystitis groups, while there were detectable levels of CXCL8, CCL2, and IL-6 in all subjects.
Figure 1. Distributions of urinary CXCL1, CXCL8, CCL2, and IL-6 in patients with acute cystitis, ASB, and negative controls. Concentrations are in pg/mg creatinine (log. scale).
shows values for sensitivity and specificity in discriminating between acute cystitis and asymptomatic bacteriuria. Values for leukocyte esterase in acute cystitis and asymptomatic bacteriuria are presented in , where they are plotted together with IL-6, illustrating diagnostic possibilities. Combined test with leukocyte esterase > 2 and IL-6 > 30 pg/mg gives a specificity of 100% and a sensitivity of 69% (95% CI 42–88) in discriminating between acute cystitis and asymptomatic bacteriuria. Among the negative controls (n = 20), 16 had no urinary leukocyte esterase, 3 had level 1, and 1 had level 2. None of the controls had a level higher than 2.
Figure 2. Urinary IL-6 (pg/mg creatinine) plotted against urinary leukocyte esterase in subjects with acute cystitis and ASB. Cut-off values (leukocyte esterase:>2, IL-6:>30pg/mg) indicated as bold lines.
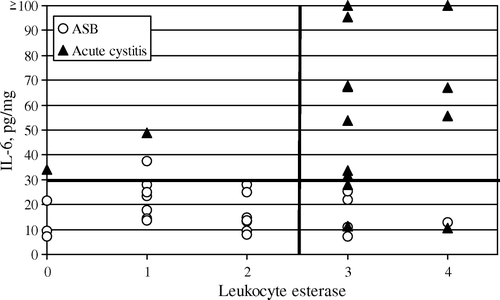
Table II. Sensitivity and specificity with 95% confidence intervals (CI) for urinary cytokines (cut-off values in pg/mg creatinine) and leukocyte esterase when discriminating between acute cystitis and ASB.
Discussion
This study revealed higher urinary levels of CXCL1, CXCL8, and IL-6 in patients with acute cystitis than in patients with ASB.
To make the results more reliable, we tried to clearly define the groups, making sure that only those subjects with distinct symptoms of infection from the lower urinary tract were included in the acute cystitis group. However, this was a small, exploratory study and the results need to be confirmed, preferably in a more clinical context.
Correction for urinary creatinine was carried out to diminish the influence of differences in diuresis, in order to create a more accurate image of real cytokine exudation into the urine. However, the fact that the differences in urinary levels of CXCL1, CXCL8, and IL-6 between the acute cystitis and ASB groups remained when testing without correction for creatinine indicates that measurement of urine cytokine levels, without this correction, could be possible in clinical practice.
In our study, the inclusion criteria for subjects with ASB were limited to those with growth of E. coli while in the acute cystitis group we found a variety of the common uropathogenic bacteria. Nevertheless, we think it is possible to compare urinary cytokine levels, as it has been shown that at least CXCL8 is elevated in UTI, independent of the causative bacteria Citation[13], Citation[16]. CXCL8 has also been shown to be highly stable in urine as a function of time and temperature Citation[16].
Except for higher levels of TNF-α and IL-12, and lower levels of IL-18 in the ASB group as compared with negative controls, we found no differences between the groups with regard to the pro-inflammatory cytokines IL-1β, TNF-α, IL-12, and IL-18, and this is in accordance with the findings of others Citation[13], Citation[22], Citation[23].
CXC-chemokines play an important role in neutrophil activation Citation[14], and urinary levels of CXCL8 correlate with the level of leukocyturia Citation[13], Citation[16], Citation[24]. There is evidence that in UTI these chemokines are produced locally in the urinary tract Citation[13], Citation[24], Citation[25]. We found gradually increasing urinary levels of CXCL1 and CXCL8 going from negative controls to ASB and on to acute cystitis. Our findings are in accordance with previous reports on younger populations Citation[9], Citation[18], Citation[25], Citation[26].
IL-6 is a pro-inflammatory and regulatory cytokine, stimulating the production of acute phase reactants such as CRP and the development of fever. We found distinctly higher urinary levels of IL-6 in acute cystitis than in ASB patients, and somewhat higher levels again in ASB patients than in negative controls. This difference, comparing UTI with ASB, has also been found in children Citation[9]. Urine IL-6 was elevated in children and adults with UTI Citation[13], Citation[17], Citation[23], Citation[27], and urinary levels of IL-6 in UTI reflected disease severity Citation[17], Citation[19], Citation[28].
With the exception of an early study by Nicolle Citation[15] this is, to our knowledge, the first study evaluating urinary cytokine response in relation to bacteriuria in an elderly population. ASB is highly prevalent in the elderly, 20% in community-living women aged 80 and over Citation[2], up to 50% in an institutionalised elderly population Citation[3], Citation[20], and the cytokine response found in our study fits into a model where ASB in this population can be regarded as an infection and not just bacterial colonization. Symptoms in UTI are caused by the inflammatory response. However, the bacteria in ASB may be less virulent and cause a milder inflammatory response Citation[29]. As cytokines/chemokines are early mediators of this inflammatory response, they are likely to be found in lower levels in patients with ASB than symptomatic UTI.
Predictive values are more useful in clinical practice than sensitivity and specificity but imply knowledge about the prevalence in the population, and thus our data do not permit such calculations. However, when the testing is bound to subjects with bacteriuria there will be a relatively high prevalence of acute cystitis and consequently the above specified values for sensitivity and specificity (see ) will result in a high positive predictive value as far as to discriminate between acute cystitis and ASB in subjects with diffuse symptomatology. Other researchers have proposed the measurement of cytokines in diagnosing febrile UTI in younger patients, with cut-off values similar to those used in our study Citation17–19.
Leukocytes in the urine are common in the elderly. In a Finnish study, the prevalence was 47% in a population aged 85 years or over, 77% in those with bacteriuria, and 37% in those without Citation[30]. Others have found leukocyturia in 90% of subjects with ASB Citation[20], but these studies do not usually distinguish among different levels of leukocyturia. Although previous studies indicate that an increase in the number of leukocytes in the urine is a non-specific finding, our results may demonstrate that with a higher cut-off value (>2) measurement of leukocyte esterase could help in distinguishing between symptomatic UTI and ASB. Further, a combination of urinary IL-6 and leukocyte esterase (see ) might increase clinical accuracy.
In conclusion, in the light of increasing rates of antibiotic resistance and high rates of antibiotic prescriptions for UTI in elderly people, it is essential to increase diagnostic accuracy to avoid overuse of antibiotics in this age group. In daily practice the first urinary test performed is often a dipstick test measuring leukocyte esterase. This study indicates that measuring leukocyte esterase and applying a higher cut-off value than is usually used may be a first step to improve the diagnostic process. In addition, a combination of leukocyte esterase and IL-6 in a dipstick in the future might be still more useful. However, the results need to be confirmed, preferably in a larger, prospective study on UTI in the elderly, evaluating the use of urinary IL-6 and leukocyte esterase in the diagnostic process.
Acknowledgements
The study was approved by the Regional Ethical Review Board of Uppsala, Sweden. Financial support for this study was provided by the county council of Dalarna, Sweden and STRAMA (Swedish Strategic Programme for the Rational Use of Antimicrobial Agents). Thanks are also offered to Marita Skarstedt for excellent technical assistance. Conflicts of interests: None.
References
- STRAMA. SWEDRES 2007. A report on Swedish antibiotic utilisation and resistance in human medicine. 2008. Available at: http://en.strama.se/dyn//,116,.html, , [accessed 20 August 2008].
- Rodhe N, Mölstad S, Englund L, Svärdsudd K. Asymptomatic bacteriuria in a population of elderly residents living in a community setting: Prevalence, characteristics and associated factors. Fam Pract 2006; 23: 303–7
- Hedin K, Petersson C, Widebäck K, Kahlmeter G, Mölstad S. Asymptomatic bacteriuria in a population of elderly in municipal institutional care. Scand J Prim Health Care 2002; 20: 166–8
- Abrutyn E, Mossey J, Levison M, Boscia J, Pitsakis P, Kaye D. Epidemiology of asymptomatic bacteriuria in elderly women. J Am Geriatr Soc 1991; 39: 388–93
- Nicolle LE. Asymptomatic bacteriuria: When to screen and when to treat. Infect Dis Clin North Am 2003; 17: 367–94
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40: 643–54
- Rodhe N, Englund L, Mölstad S, Samuelsson E. Bacteriuria is associated with urge urinary incontinence in older women. Scand J Prim Health Care 2008; 26: 35–9
- Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J 1990; 265: 621–36
- Benson M, Jodal U, Agace W, Hellstrom M, Marild S, Rosberg S, et al. Interleukin (IL)-6 and IL-8 in children with febrile UTI and asymptomatic bacteriuria. J Infect Dis 1996; 174: 1080–4
- Funfstuck R, Franke S, Hellberg M, Ott U, Knofel B, Straube E, et al. Secretion of cytokines by uroepithelial cells stimulated by Escherichia coli and Citrobacter spp. Int J Antimicrob Agents 2001; 17: 253–8
- Hedges S, Stenqvist K, Lidin-Janson G, Martinell J, Sandberg T, Svanborg C. Comparison of urine and serum concentrations of interleukin-6 in women with acute pyelonephritis or asymptomatic bacteriuria. J Infect Dis 1992; 166: 653–6
- Jantausch BA, O'Donnell R, Wiedermann BL. Urinary interleukin-6 and interleukin-8 in children with urinary tract infection. Pediatr Nephrol 2000; 15: 236–40
- Ko YC, Mukaida N, Ishiyama S, Tokue A, Kawai T, Matsushima K, et al. Elevated interleukin-8 levels in the urine of patients with urinary tract infections. Infect Immun 1993; 61: 1307–14
- Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med 1998; 338: 436–45
- Nicolle LE, Brunka J, Orr P, Wilkins J, Harding GK. Urinary immunoreactive interleukin-1 alpha and interleukin-6 in bacteriuric institutionalized elderly subjects. J Urol 1993; 149: 1049–53
- Oregioni O, Delaunay P, Bruna P, Gaudart A, Lemichez E, Boquet P, et al. Urinary interleukin-8 is elevated in urinary tract infections independently of the causative germs. Cytokine 2005; 31: 415–18
- Otto G, Braconier J, Andreasson A, Svanborg C. Interleukin-6 and disease severity in patients with bacteremic and nonbacteremic febrile urinary tract infection. J Infect Dis 1999; 179: 172–9
- Otto G, Burdick M, Strieter R, Godaly G. Chemokine response to febrile urinary tract infection. Kidney Int 2005; 68: 62–70
- Sheu JN, Chen MC, Lue KH, Cheng SL, Lee IC, Chen SM, et al. Serum and urine levels of interleukin-6 and interleukin-8 in children with acute pyelonephritis. Cytokine 2006; 36: 276–82
- Nicolle LE. Asymptomatic bacteriuria in the elderly. Infect Dis Clin North Am 1997; 11: 647–62
- Rodgers K, Nicolle LE, McIntyre M, Harding GK, Hoban D, Murray D. Pyuria in institutionalized elderly subjects. Can J Infect Dis 1991; 2: 142–6
- Kassir K, Vargas-Shiraishi O, Zaldivar F, Berman M, Singh J, Arrieta A. Cytokine profiles of pediatric patients treated with antibiotics for pyelonephritis: potential therapeutic impact. Clin Diagn Lab Immunol 2001; 8: 1060–3
- Benson M, Jodal U, Andreasson A, Karlsson A, Rydberg J, Svanborg C. Interleukin 6 response to urinary tract infection in childhood. Pediatr Infect Dis J 1994; 13: 612–16
- Agace WW, Hedges SR, Ceska M, Svanborg C. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J Clin Invest 1993; 92: 780–5
- Olszyna DP, Opal SM, Prins JM, Horn DL, Speelman P, van Deventer SJ, et al. Chemotactic activity of CXC chemokines interleukin-8, growth-related oncogene-alpha, and epithelial cell-derived neutrophil-activating protein-78 in urine of patients with urosepsis. J Infect Dis 2000; 182: 1731–7
- Real JM, Munro P, Buisson-Touati C, Lemichez E, Boquet P, Landraud L. Specificity of immunomodulator secretion in urinary samples in response to infection by alpha-hemolysin and CNF1 bearing uropathogenic Escherichia coli. Cytokine 2007; 37: 22–5
- Jacobson SH, Hylander B, Wretlind B, Brauner A. Interleukin-6 and interleukin-8 in serum and urine in patients with acute pyelonephritis in relation to bacterial-virulence-associated traits and renal function. Nephron 1994; 67: 172–9
- Krzemien G, Roszkowska-Blaim M, Kostro I, Szmigielska A, Karpinska M, Sieniawska M, et al. Urinary levels of interleukin-6 and interleukin-8 in children with urinary tract infections to age 2. Med Sci Monit 2004; 10: CR593–7
- Svanborg C, Godaly G. Bacterial virulence in urinary tract infection. Infect Dis Clin North Am 1997; 11: 513–29
- Heinamaki P, Haavisto M, Mattila K, Rajala S. Urinary characteristics and infection in the very aged. Gerontology 1984; 30: 403–7