Abstract
Objective: This study aims to assess potentially severe class D drug–drug interactions (DDDIs) in residents 65 years or older in assisted living facilities with the use of a Swedish and Finnish drug–drug interaction database (SFINX).
Design: A cross-sectional study of residents in assisted living facilities in Helsinki, Finland.
Setting: A total of 1327 residents were assessed in this study. Drugs were classified according to the Anatomical Therapeutic Chemical (ATC) classification system and DDDIs were coded according to the SFINX.
Main outcome measures: Prevalence of DDDIs, associated factors and 3-year mortality among residents.
Results: Of the participants (mean age was 82.7 years, 78.3% were females), 5.9% (N = 78) are at risk for DDDIs, with a total of 86 interactions. Participants with DDDIs had been prescribed a higher number of drugs (10.8 (SD 3.8) vs. 7.9 (SD 3.7), p < 0.001). A larger proportion of residents with DDDIs suffered from rheumatoid arthritis or osteoarthritis than those not exposed to DDDIs (24.7% vs. 15.4%, p = 0.030). The most frequent DDDIs were related to the concomitant use of potassium with amiloride (N = 12) or spironolactone (N = 12). Carbamazepine (N = 13) and methotrexate (N = 9) treatments were also frequently linked to DDDIs. During the follow-up, no differences in mortality emerged between the participants exposed to DDDIs and the participants not exposed to DDDIs.
Conclusions: Of the residents in assisted living, 5.9% were exposed to DDDIs associated with the use of a higher number of drugs. Physicians should be trained to find safer alternatives to drugs associated with DDDIs.
Potentially severe, class D drug–drug interactions (DDDIs) have been defined in the SFINX database as clinically relevant drug interactions that should be avoided.
• Of the residents in assisted living, 5.9% were exposed to DDDIs that were associated with the use of a higher number of drugs.
• The most frequent DDDIs were related to the concomitant use of potassium with amiloride or spironolactone. Carbamazepine and methotrexate were also linked to DDDIs.
• No difference in mortality was observed between residents exposed to DDDIs and residents not exposed to DDDIs.
KEY POINTS
Introduction
Older people in assisted living facilities are prone to potentially severe drug–drug interactions (DDIs) due to comorbidities and the use of a higher number of drugs.[Citation1] Potentially severe DDIs correspond to class D interactions (DDDIs) according to the Swedish, Finnish Interaction X-referencing (SFINX) database. They may lead to negative clinical outcomes and should always be avoided. They have received increasing attention in older populations since multiple medication use is becoming more common in managing diseases. DDIs have been studied in hospital and outpatient settings, and from drug registers. Register-based studies are the most common, reporting DDI prevalence rates of 15–26%,[Citation2–4] whereas studies conducted in community settings show prevalence of 26.5–63%.[Citation5,Citation6] Prevalence rates also vary in hospital settings, including wards (57.8%) [Citation7] and emergency departments (0.7%).[Citation8] The definition for DDIs has varied from one study to another depending on the applied assessment methods, populations and study settings, thus resulting in a wide range of prevalence. This makes it difficult to compare DDIs between studies. Few researchers have evaluated the severity of DDDIs. To our knowledge, limited studies have additionally examined the prevalence rates of DDDIs among institutionalized residents (4.8%),[Citation1] who are most susceptible to the use of a higher number of drugs [Citation9] and, therefore, also to DDDIs.[Citation3–6] Irrespective of the setting, DDDIs have been evaluated to be less common (0.7–16%).[Citation1,Citation3–6,Citation8]
DDDIs have been shown to associate with patients’ increasing age,[Citation4,Citation10,Citation11] female gender,[Citation12] the use of a higher number of drugs [Citation3–6,Citation11–13] and cardiovascular diseases.[Citation12] The most frequently reported DDDIs have been associated with the use of anticoagulants,[Citation10,Citation13] potassium sparing-diuretics, potassium supplements, or ACE inhibitors [Citation1,Citation14] and carbamazepine.[Citation1] DDDIs are related to adverse events, an increased number of hospitalizations [Citation14,Citation15] and higher healthcare costs.[Citation15]
DDDIs can be predicted and avoided through education and interventions.[Citation3,Citation16,Citation17] Several studies have shown that physicians are only aware of a minority of DDDIs.[Citation16,Citation18]
Although several large register-based studies have reported DDDI prevalence, few studies to date have examined the clinical outcomes of DDDIs in frail, older populations prone to using a higher number of drugs. We hypothesized that DDDIs are associated with the use of a higher number of drugs, comorbidities and increased mortality. The aim of this study was to describe (1) the prevalence of DDDIs according to the SFINX database among older people living in residential care facilities and associated characteristics of residents and (2) to compare the mortality of residents with and without DDDIs.
Materials and methods
Settings and study population
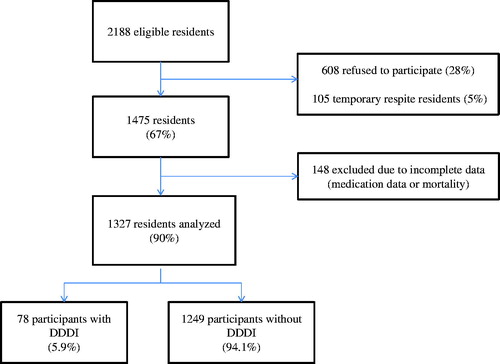
A cross-sectional study was carried out among older people in all residential care facilities in the cities of Helsinki and Espoo, Finland, based on data collected in February 2007 as part of a larger project investigating nutritional status and nutritional care.[Citation19] The study includes all 36 and 33 residential care units located in Helsinki and Espoo, respectively. Of the eligible residents (N = 2188), 67% (N = 1475) consented to participate in the study. Nonparticipants either refused (28%, N = 608) or were residents in temporary respite care (5%, N = 105) (). Of all residents, 148 were excluded due to complete medication or mortality data being unavailable. Data were thus available for a total of 1327 residents. The assisted living facilities in Helsinki and Espoo provide round-the-clock care with a registered nurse in charge, similar to traditional nursing homes. However, the environment in assisted living units is more home-like than in traditional nursing homes. Resident characteristics are similar to those in traditional nursing homes.[Citation19]
Assessments
Health status data, demographic factors, drug use, and diagnoses were retrieved from medical charts by nurses working in care units, who had received specific training by researchers for gathering the medical data. All assessments and data gathering were performed during a single day (31 March 2007). Mortality dates were retrieved from the Finnish central registers until 6 July 2010.
The Charlson comorbidity index was used, taking into account the number and severity of comorbid conditions.[Citation20] The Clinical Dementia Scale (CDR) was used to assess the cognitive state of the participants.[Citation20] Dependency in activities of daily living (ADL) was defined as “requiring at least prompting or assistance in dressing, hygiene, managing personal effects, or requiring much help with personal care, often involving incontinence’’ in the “personal care” item of the Clinical Dementia Rating scale (CDR class 1 or higher).[Citation21]
Residents were classified using the Mini Nutritional Assessment (MNA) test,[Citation22] in the following ways: (i) good nutritional status, MNA score 24–30, (ii) risk of malnutrition, MNA score 17–23.5, and (iii) malnutrition, MNA score <17.
Psychological well-being (PWB) was assessed using the PWB score,[Citation23] which is derived from six questions: (1) “Are you satisfied with your life?” (yes/no), (2) “Do you have zest for life?” (yes/no), (3) “Do you have plans for the future?” (yes/no), (4) “Do you feel needed?” (yes/no), (5) “Do you feel depressed?” (seldom or never/sometimes/often or always), and (6) “Do you suffer from loneliness?” (seldom or never/sometimes/often or always). The score is created so that each question represents 0 (“no” in questions 1–4, “often or always” in questions 5 and 6), 0.5 (“sometimes” in questions 5 and 6) or 1 point (“yes” in questions 1–4, “seldom or never” in questions 5 and 6). The score is calculated by dividing the total points with the number of questions answered by the participant. A score of 1 thus represents the best and 0 the poorest PWB. A difference of 0.08–0.1 in scale can be considered clinically meaningful. The score has been validated among older people.[Citation23]
The use of medications was assessed as a point-prevalence during the assessment day. Residents were classified as regular drug users if their medical charts indicated a regular sequence for drug dosage. Only drugs used on a regular basis were taken into account. We did not have information on how often the participants used pro re nata drugs so they were excluded. All drugs were classified according to the Anatomical Therapeutic Chemical (ATC) classification system (WHO Collaborating Center for Drug Statistics Methodology 2010).
The SFINX computerized database system [Citation24] was used for assessing DDDIs. SFINX is a commercial drug–drug interaction database and software providing short and concise evidence based information concerning the consequences of and recommendations for ∼18,000 drug combinations. It has been commonly used by Finnish doctors since 2005 and is updated four times a year by Medbase Ltd in Turku, Finland, the Karolinska Institute Department of Clinical Pharmacology in Stockholm, and the Stockholm County Council, Sweden. Interactions are classified according to their clinical significance (A–D) and documentation level (0–4), where A indicates a clinically insignificant interaction and D a clinically significant interaction that should be avoided. The database does not automatically alert the physician; potential interactions must be checked individually.[Citation24]
Outcomes
The number of DDDIs and mortality were the main outcomes. We considered comorbidities, different medical conditions, the number of medications, functioning, nutrition and PWB to be potential associates and confounders of the main outcome measures.
Statistical methods
For data analysis, the Number Cruncher Statistical System (NCSS) (www.ncss.com) and SPSS 12.0.1 (SPSS Inc., Chicago, IL) software programs were applied. Differences in proportions were tested with the X2 – test. The Mann–Whitney U-test was used for evaluating non-normally distributed continuous variables in this study. In all analyses, p < 0.05 was considered statistically significant. The Helsinki University Central Hospital Ethics Committee approved the study protocol. Informed consent was acquired from each participant.
Results
Residents’ mean age was 82.7 years (SD 7.8), and 78.3% were females. A mean of 8.0 (SD 2.9) drugs were administered regularly to each resident.
Of the participants, 5.9% (N = 78) run the risk of DDDIs, with a total of 86 interactions. Eight residents were susceptible to two DDDIs.
More often than other residents, those exposed to DDDIs had been prescribed a higher number of drugs (10.8 (SD 3.8) vs. 7.9 (SD 3.7), p < 0.001) and had arthritis. Residents exposed to DDDIs showed no significant differences in demographic characteristics, common medical conditions, the Charlson comorbidity index, malnutrition, cognitive impairment, mobility, functional ability or PWB compared with those not exposed to DDDIs (). An association trend was observed between cardiovascular diseases and DDDIs (p = 0.070).
Table 1. Characteristics of residents in assisted living divided according to their exposure to all class D drug–drug interactions (DDIs).
The most frequent DDDIs were related to the concomitant use of potassium and either amiloride (N = 12) or spironolactone (N = 12). However, 12 residents concomitantly using potassium and potassium-sparing diuretics were also administered furosemide. We also found class DDDIs with the concomitant use of carbamazepine and risperidone (N = 5), felodipin (N = 2), ciclosporin (N = 1), quetiapine (N = 1), estriol (N = 1), oxycodone (N = 1), tolterodine (N = 1), or lercanidipine (N = 1). The concomitant use of methotrexate and pantoprazole (N = 4), omeprazole (N = 2), esomeprazole (N = 2), or lansoprazole (N = 1) was also reported. The concomitant use of a calcium-channel and beta-blockers was observed in 10 residents. Only three DDDI cases caused by concomitant use of nonsteroidal anti-inflammatory drugs (NSAID) and warfarin were found ().
Table 2. Class D drug–drug interactions (DDIs) in assisted living residents in Helsinki and Espoo, Finland.
No differences emerged in the three-year all-cause mortality between the residents exposed to DDDIs and those not exposed to DDDIs. (46.2% vs. 44.4%, p = 0.76) ().
Discussion
Of the participating residents in assisted living facilities, one in 17 had a combination of drugs that should be avoided, and are thus at risk for class D interactions. Patients prescribed a higher number of drugs or with arthritis were associated with more DDDI combinations. The most commonly identified interacting medication pairs were potassium with potassium-sparing diuretics, methotrexate with proton-pump inhibitors, carbamazepine with various drugs, and calcium-channel blockers with beta-blockers. DDDIs were not associated with higher mortality.
The main strength of this study is its large and representative geriatric population in an institutionalized setting with high levels of comorbidities, prevalent dementia, and the use of a large number of drugs. The nurses who retrieved the clinical data for the study had a specific training for the purpose. That strengthens the validity of the study. One limitation of the study is its cross-sectional nature, which does not allow drawing conclusions on causal relationships between DDDIs and associated factors. The participation rate of this study was 67%, which is well in line with comparable studies. Unfortunately, we do not have information concerning the nonparticipants. Informed consent could often not be obtained from this group due to severe dementia and not having a close proxy. Another limitation is that we have no clinical data on actual adverse events or hospitalizations. Nor we had access to medication records after the time of this study. Possible changes of medication could have brought about DDDIs and could also have been associated with mortality. Furthermore, the generalizability of our study is limited to older people’s care facilities.
The prevalence of DDDIs (5.9%) falls between the lowest and highest rates presented in earlier studies (0.7–16%).[Citation1,Citation3,Citation4,Citation6,Citation8] Two of these studies have used exactly the same criteria (SFINX) for DDDIs,[Citation1,Citation3] and showed strikingly similar prevalence rates of 5% and 4.8%. Johnell and Klarin’s was a register-based study in a general older population whereas the study of Hosia-Randell et al. was performed in an institutional setting. That study showed that the most common DDDIs were related to the use of potassium-sparing diuretics, carbamazepine, and codeine. Compared with residents not exposed to DDDIs, those exposed to potential DDDIs were more likely to be younger, to have a prior history of stroke, to be taking psychotropics, to be administered nine or more drugs daily and to be taking potentially inappropriate drugs.[Citation1]
Our results are in line with earlier studies concluding that a higher number of prescribed drugs increases the risk of DDDIs.[Citation1–5,Citation11–13] Most studies have assessed DDDIs but not their outcomes. Only a few studies have explored the adverse events as consequences of DDDIs.[Citation14,Citation15,Citation24] To our knowledge, our study is the first one to investigate the association between DDDIs and mortality. It is somewhat surprising that no association was observed. Even though mortality is high in a study population like ours, our study is underpowered to detect small differences in mortality between those with DDDIs and those without.
Cardiovascular diseases [Citation12] and stroke [Citation1] have been associated with a higher DDDI risk. However, we found no association between cardiovascular diseases or stroke and DDDIs in our sample, although a trend (p = 0.07) emerged between coronary heart disease and DDDIs. This may have clinical implications since cardiovascular diseases are so common in frail populations.
The most common DDDIs in previous studies have been associated with the use of anticoagulants,[Citation10,Citation13,Citation25] potassium-sparing diuretics, potassium supplements, or angiotensin-converting enzyme (ACE) inhibitors,[Citation1,Citation3,Citation14] aspirin with other NSAIDs [Citation3] and carbamazepine with various drugs.[Citation1] Our findings confirm that the concomitant use of potassium-sparing diuretics and potassium supplements are common DDDIs. Among concomitant users of potassium supplements and potassium-sparing agents in our study, 12 of them were also administered furosemide, which might lower potassium levels. This suggests that the physicians prescribing these drugs may be aware of these DDDIs. Close potassium follow-up is common among Finnish residential care patients to prevent hyperkalemia, and clinical benefits may thus overshadow the potential risks of DDDIs.[Citation8] It is important to estimate whether the benefit of a combination of drugs outweighs the potential risk of an adverse effect. In line with a previous study,[Citation1] carbamazepine commonly predisposed users to several DDDIs. However, DDDIs related to the use of anticoagulants was fairly uncommon in our population.
Our findings suggest that the use of methotrexate with proton pump-inhibitors (PPIs) may also commonly predispose users to DDDIs. To our knowledge, this is a new finding, although the seriousness of the interaction potential has been discussed.[Citation1,Citation26] Autoimmune diseases are common among older people, and methotrexate has become a common treatment of these diseases. The small methotrexate doses used in autoimmune diseases and arthritis may not have clinically significant interaction with PPIs. The concomitant use of high methotrexate doses and PPIs is considered potentially dangerous. None of our participants used high-dose methotrexate for cancer. The use of PPIs is very prevalent in institutionalized settings and they are often used along with NSAIDs.[Citation27] Thus, rheumatoid arthritis may just be a confounder associated with the use of both methotrexate and NSAIDs with concomitant PPIs.
DDDI prevalence varies between countries, settings, and populations. The most common drugs involved in DDDI also seem to vary over time. Our study suggests that, e.g. in Finnish institutional settings DDDIs related to codeine have disappeared between the years 2003 and 2007 [Citation1], whereas potassium-sparing diuretics and carbamazepine continue to predispose patients to DDDIs.[Citation1]
The prevalence of DDDIs remained very similar between the years 2003 and 2007 in Finland, despite the introduction of the SFINX database during this period. Automatically alerting software programmes have been set up for DDDIs, many of which are integrated into clinical decision-support programmes hopefully contributing to improved healthcare and decreased costs in older patients.[Citation28] In Sweden, DDDIs were associated with a 17% decrease of interactions from 2.15 × 10−3 to 1.81 × 10−3 interactions per prescribed drug–drug pair by integrating the SFINX database into primary care electronic health records.[Citation17] Our study suggests that Finnish physicians do not fully take advantage of SFINX, which is available to them but does not automatically alert them. It has been gradually introduced to physicians and assisted living facilities since 2005. A study from today, 2015, could result in different findings, as the SFINX system is currently more widely used. However, computerized drug prescribing alerts may improve patient safety, but are often overridden because of poor specificity and alert overload.[Citation29–31] At the time of our study, the assisted living facilities in Helsinki were most often consulted by temporarily hired physicians. As suggested by geriatricians in their survey, these old, multimorbid residents would probably benefit from care by GPs working continuously with them.[Citation32]
Conclusions
About 6% of frail older people in residential care facilities were exposed to potentially most severe class D interactions (DDDIs). This exposure was associated with the use of a high number of drugs, but not with all-cause mortality or with the degree of psychological wellbeing. The introduction of drug–drug interaction database systems has not reduced the prevalence of DDDIs among residents of assisted living facilities in 2007. Further studies are needed to investigate physicians’ knowledge of and attitudes towards DDDIs and also the usability of the computerized database system SFINX for assessing DDDIs.
Funding information
This study was supported by The Finnish Medical Association and Uulo Arhio Foundation.
Disclosure statement
The authors report no conflict of interest related to this study. The authors alone are responsible for the content and writing of the paper.
References
- Hosia-Randell HM, Muurinen SM, Pitkälä KH. Exposure to potentially inappropriate drugs and drug–drug interactions in elderly nursing home residents in Helsinki, Finland: a cross-sectional study. Drugs Aging. 2008;25:683–692.
- Bjerrum L, Andersen M, Petersen G, et al. Exposure to potential drug interactions in primary health care. Scand J Prim Health Care. 2003;21:153–158.
- Johnell K, Klarin I. The relationship between number of drugs and potential drug–drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish prescribed drug register. Drug Saf. 2007;30:911–918.
- Nobili A, Pasina L, Tettamanti M, et al. Potentially severe drug interactions in elderly outpatients: results of an observational study of an administrative prescription database. J Clin Pharm Ther. 2009;34:377–386.
- Secoli SR, Figueras A, Lebrão ML, et al. Risk of potential drug–drug interactions among Brazilian elderly: a population-based, cross-sectional study. Drugs Aging. 2010;27:759–770.
- Teixeira JJ, Crozatti MT, dos Santos CA, et al. Potential drug–drug interactions in prescriptions to patients over 45 years of age in primary care, southern Brazil. PLoS One. 2012;7:e47062.
- Janchawee B, Owatranporn T, Mahatthanatrakul W, et al. Clinical drug interactions in outpatients of a university hospital in Thailand. J Clin Pharm Ther. 2005;30:583–590.
- Bakken MS, Ranhoff AH, Engeland A, et al. Inappropriate prescribing for older people admitted to an intermediate-care nursing home unit and hospital wards . Scand J Prim Health Care. 2012;30:169–175.
- Jyrkkä J, Vartiainen L, Hartikainen S, et al. Increasing use of medicines in elderly persons: a five-year follow-up of the Kuopio 75 + study. Eur J Clin Pharmacol. 2006;62:151–158.
- Malone DC, Hutchins DS, Haupert H, et al. Assessment of potential drug–drug interactions with a prescription claims database. Am J Health Syst Pharm. 2005;62:1983–1991.
- Lin CF, Wang CY, Bai CH. Polypharmacy, aging and potential drug–drug interactions in outpatients in Taiwan: a retrospective computerized screening study. Drugs Aging. 2011;28:219–225.
- Cruciol-Souza JM, Thomson JC. Prevalence of potential drug–drug interactions and its associated factors in a Brazilian teaching hospital. J Pharm Pharm Sci. 2006;9:427–433.
- Zhan C, Correa-de-Araujo R, Bierman AS, et al. Suboptimal prescribing in elderly outpatients: potentially harmful drug–drug and drug-disease combinations. J Am Geriatr Soc. 2005;53:262–267.
- Juurlink DN, Mamdani M, Kopp A, et al. Drug–drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652–1658.
- Moura CS, Acurcio FA, Belo NO. Drug–drug interactions associated with length of stay and cost of hospitalization. J Pharm Pharm Sci. 2009;12:266–272.
- Langdorf MI, Fox JC, Marwah RS, et al. Physician versus computer knowledge of potential drug interactions in the emergency department. Acad Emerg Med. 2000;7:1321–1329.
- Andersson ML, Böttiger Y, Lindh JD, et al. Impact of the drug–drug interaction database SFINX on prevalence of potentially serious drug–drug interactions in primary health care. Eur J Clin Pharmacol. 2013;69:565–571.
- Ko Y, Malone DC, D'Agostino JV, et al. Potential determinants of prescribers’ drug–drug interaction knowledge. Res Social Adm Pharm. 2008;4:355–366.
- Muurinen SM, Soini HH, Suominen MH, et al. Vision impairment and nutritional status among older assisted living residents. Arch Gerontol Geriatr. 2014;58:384–387.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572.
- Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition the mini nutritional assessment. Clin Geriatr Med. 2002;18:737–757.
- Routasalo PE, Tilvis RS, Kautiainen H, et al. Effects of psychosocial group rehabilitation on social functioning, loneliness and well-being of lonely, older people: randomized controlled trial. J Adv Nurs. 2009;65:297–305.
- Böttiger Y, Laine K, Andersson ML, et al. SFINX-a drug–drug interaction database designed for clinical decision support systems. Eur J Clin Pharmacol. 2009;65:627–633.
- Bucşa C, Farcaş A, Cazacu I, et al. How many potential drug–drug interactions cause adverse drug reactions in hospitalized patients? Eur J Intern Med. 2013;24:27–33.
- Delafuente JC. Understanding and preventing drug interactions in elderly patients. Crit Rev Oncol Hematol. 2003;48:133–143.
- Teramura-Grönblad M, Bell JS, Pöysti MM, et al. Risk of death associated with use of PPIs in three cohorts of institutionalized older people in Finland. J Am Med Dir Assoc. 2012;13:488.e9–488.e13.
- Tamblyn R, Reidel K, Patel V. Physicians’ response to computerised alerts for psychotropic drugs in older persons: a multilevel analysis of the associated alert, patient and physician characteristics. BMJ Open. 2012;19:635–643.
- Ash JS, Berg M, Coiera E. Some unintended consequences of information technology in health care: the nature of patient care information system-related errors. J Am Med Inform Assoc. 2004;11:104–112.
- Shah NR, Seger AC, Seger DL, et al. Improving acceptance of computerized prescribing alerts in ambulatory care. J Am Med Inform Assoc. 2006;13:5–11.
- Isaac T, Weissman JS, Davis RB, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med. 2009;169:305–311.
- Löppönen M, Heinonen P, Jartti L, et al. The appreciation of geriatricians has improved but ability to influence decision-making has declined. (In Finnish). Finnish Med J. 2014;69:991–992.