Abstract
Objective: Internet-delivered cognitive behavioral therapy (ICBT) is recommended as an efficient treatment alternative for depression in primary care. However, only few previous studies have been conducted at primary care centers (PCCs). We evaluated long-term effects of ICBT treatment for depression compared to treatment as usual (TAU) in primary care settings.
Design: Randomized controlled trial.
Setting: Patients were enrolled at16 PCCs in south-west Sweden.
Participants: Patients attending PCCs and diagnosed with depression (n = 90).
Interventions: Patients were assessed by a primary care psychologist/psychotherapist and randomized to ICBT or TAU. The ICBT included an ICBT program consisting of seven modules and weekly therapist e-mail or telephone support during the 3-month treatment period.
Main outcome measures: Questionnaires on depressive symptoms (BDI-II), quality of life (EQ-5D) and psychological distress (GHQ-12) were administered at baseline, with follow-ups at 3, 6 and 12 months. Antidepressants and sedatives use, sick leave and PCC contacts were registered.
Results: Intra-individual change in depressive symptoms did not differ between the ICBT group and the TAU group during the treatment period or across the follow-up periods. At 3-month follow-up, significantly fewer patients in ICBT were on antidepressants. However, the difference leveled out at later follow-ups. There were no differences between the groups concerning psychological distress, sick leave or quality of life, except for a larger improvement in quality of life in the TAU group during the 0- to 6-month period.
Conclusions: ICBT with weekly minimal therapist support in primary care can be equally effective as TAU among depressed patients also over a 12-month period.
Clinical trial registration: The trial was registered in the Swedish Registry, researchweb.org, ID number 30511.
Introduction
Depression is a common mental disorder. The WHO rates depression as the leading cause of disability worldwide and as a major contributor to the global burden of disease [Citation1]. Depression is also a health problem that can exacerbate the condition of patients with comorbidity, such as diabetes and other illnesses where lifestyle behaviors have a large impact. Furthermore, depression is also a common and frequent reason for reduced work ability and sick leave, as depression is likely to contribute to functional impairment of varying severity [Citation2,Citation3].
In Sweden, as in the majority of Western countries, most patients with depression are treated in primary care. Psychological treatment with cognitive behavioral therapy (CBT) is currently recommended as the first choice for treating mild depression and one of the first choices for treating moderate depression [Citation4]. Internet-delivered cognitive behavioral therapy (ICBT) treatment has been evaluated in a number of comparative studies [Citation5,Citation6] and is currently proposed as a treatment alternative to face-to-face CBT also in primary care [Citation7,Citation8]. ICBT allows greater access, and possibly more effective health care (e.g. shorter therapist time spent per patient), but more research is needed to evaluate ICBT in primary care settings [Citation9]. Several studies show that ICBT reduces symptoms of depression, and the effect is persistent in long-term follow-up [Citation10,Citation11]. However, patients have mostly been recruited through advertisements or specialized clinics, and only few ICBT studies have been conducted in ordinary primary care [Citation9].
In addition, in ICBT research, performed interventions are often diverse; including various types of software programs, computerized CBT on site at clinics, live-CBT online and unsupported ICBT, which complicates comparisons [Citation5,Citation6,Citation12,Citation13]. The effectiveness of ICBT in primary care cannot be evaluated based on studies with patients via advertisement recruitment or from specialized clinics [Citation9,Citation14]. More extensive and longer follow-ups are also needed [Citation15].
The overall aim of the PRIM-NET study was to evaluate whether ICBT can improve treatment of mild/moderate depression in the primary care setting, with emphasis on long-term (6 months and 12 months) effects on depressive symptoms, as well as on quality of life, psychological distress, medication and sick leave. The specific hypothesis was that ICBT is at least equally effective, or non-inferior, compared to treatment as usual (TAU) for reduction of depressive symptoms in a long-term perspective in a primary care context. The primary outcome measure was reduction of depressive symptoms. Secondary outcomes were improvement in quality of life and reduction of psychological distress, sustained effect on depressive symptoms (6 and 12 months), and whether the need of medication and sick leave differed between treatment groups. All outcome measures are both in a short- and long-term perspective. Short-term results (3-month follow-up) concerning outcomes of depressive symptoms have been reported earlier [Citation16].
Material and methods
The PRIM-NET project was designed as a randomized controlled trial in primary care. A total of 90 patients were enrolled between March 2010 and March 2013 at 16 primary care centers (PCCs) located in the south-west region of Sweden. All patients were assessed by a psychologist/psychotherapist (therapist) and randomized to either ICBT or TAU. The ICBT in PRIM-NET included a commercially available ICBT program, together with weekly therapist e-mail or telephone support from the same therapist who had performed the initial assessment. The therapist followed the patient’s progress actively, communicated with the patient every week via a secure e-mail and encouraged the patient to provide feedback about experienced progress. The study protocol has been described in detail previously [Citation16].
PCCs
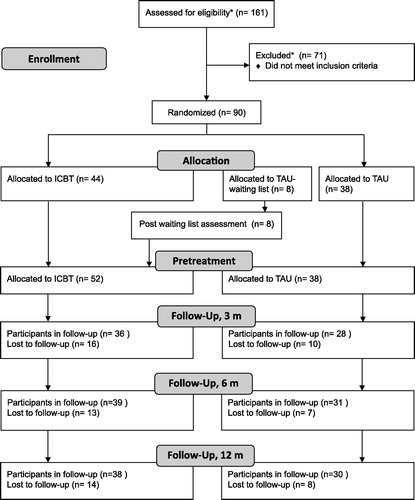
All PCCs in the study area with primary care therapists with CBT training were invited to participate in the study. Each participating PCC assigned a study nurse who was responsible for the collection of research data. PRIM-NET employed therapists with CBT training to work with PRIM-NET at two PCCs. Participating nurses, GPs and therapists were specially trained in the PRIM-NET project to perform all required activities according to a structured study protocol concerning routines for recruitment, assessment and treatment. Few of the PCCs offered ICBT for depression before the trial. The PCCs remained responsible for the patients’ treatment during the entire study period. Seven of the 16 participating PCCs were connected to a centralized primary care ICBT unit, which was set up and operated by PRIM-NET to enhance the enrolment after the first year. The ICBT unit served the seven PCCs with all parts of the study protocol after the GP consultation, except TAU, which the patients received at their PCC. All included patients randomized to TAU at the centralized unit (n = 8) were offered ICBT after the treatment period (of 3 months) for recruitment reasons (referral reinforcement). presents a flowchart of the recruitment of patients and dropouts.
Figure 1. Flowchart showing recruitment and dropouts in the PRIM-NET study. Post-waiting list assessment: eight participants, initially randomized to TAU, were included in the intervention arm for recruitment reasons and thus followed up for 12 months after start ICBT. *Uncertain number due to local inconsistencies.
Procedure
Patients
Patients aged 18 years and older with symptoms of depression who attended the study PCCs were recruited by the GPs and nurses. Patients positive to ICBT as a treatment option, who had not recently (last month) started or changed possible antidepressant medication were asked about their willingness to participate in the study. Before inclusion, the patients were invited to a therapist for a 1-h interview including a diagnostic process assessing whether the patients actually met the inclusion criteria. The diagnostic process was conducted using structured assessment interviews and validated instruments and scales, i.e. the Mini-International Neuropsychiatric Interview (MINI) version 6, MINI plus with additions from the sections concerning depression and dysthymia [Citation17], Beck Depression Inventory II (BDI-II) [Citation18] and the Montgomery–Åsberg Depression Rating Scale – self rating version (MADRS-S) [Citation19].
To be included, patients had to meet diagnostic criteria for depression according to DSM-IV (assessed via MINI), have a MADRS-S score below 35, and have access to a computer with speakers or headphones. Patients were excluded if they had severe depression (according to a MADRS-S score ≥35), a principal diagnosis of anxiety (assessed by the therapist), psychosis, bipolar disorder or hypomanic episode, antisocial personality disorder, substance dependence or alcohol abuse (all of the above assessed by therapists using MINI), medium or high suicide risk (defined as MADRS-S question 9 > 3p and/or MINI Part B – Suicide >9p, or previous suicide attempt). Patients with other severe mental disorder, cognitive disability or communication difficulties that would prevent participation in the ICBT program (only available in Swedish) were also excluded.
Randomization
Randomization took place after the diagnostic interview, when the study nurse also administered questionnaires, including background data. The patients were consecutively randomized to either ICBT or TAU by an independent research unit at the University of Gothenburg/Sahlgrenska University Hospital. The randomization process was performed with all study patients as one group, which concealed the allocation to be from both the PCC personnel and the researchers.
After the treatment period, all patients met the study nurse at the PCC for a 3-month follow-up. Evaluation with the same instruments was done after 6 and 12 months by sending the questionnaires by e-mail.
Eight participants, initially randomized to TAU, were included in the intervention arm after 3 months as TAU patients. They then received ICBT intervention and were followed up 0–12 months as ICBT patients for recruitment reasons. In the present long-term follow-up study, they were consequently regarded as ICBT patients.
Power calculation
The study was designed for detecting a possible significant difference in effect size in the summation of scores employed in the completed instruments of approximately 10% between the ICBT group and the TAU group, with significance level set at 0.05 and power estimate of 0.80, which required 71 participants in each group. The effect size was chosen based on findings reported in a study by Proudfoot et al. [Citation20] and on the study group’s empirical expectations.
Therapists
In total, 14 therapists were involved in the study. They were all licensed psychologists or licensed psychotherapists, except for two psychologists under supervision to be licensed. They all had training in CBT and had qualified knowledge and previous experience of treatment of depression. The therapists were introduced to the aim, design and procedures of the project, and the two study psychologists supervised them regularly. The study psychologists were supervised by a psychologist and instructor in CBT at the Department of Psychology at the University of Gothenburg. In all, 13 therapists performed the initial assessment. Ten therapists were engaged in the support of patients randomized to the ICBT treatment.
Intervention
ICBT
Patients randomized to ICBT received a personal login code, a printed workbook and printed guidance on how to access the treatment and the secure e-mail service from the study nurse. The ICBT program Depressionshjälpen® [Citation21] was based on a structured CBT approach, with strong emphasis on behavioral activation [Citation22] and components of acceptance and commitment therapy [Citation23]. The program consisted of seven modules, which the patients could access from a webpage using their unique personal code. The therapists could actively follow the patients’ work and progress in the program every week in a secure e-mail system called ‘Mina vårdkontakter’ (My Healthcare Contacts) (MVK), already in use within Swedish primary care. Thus, patients received support from the same therapist who had performed the initial assessment at the PCC. Contact was also made by three telephone calls during the treatment period of 8–12 weeks, plus additional contact via e-mail or telephone when needed (although rarely asked for). Manuals for the therapists were developed by PRIM-NET.
TAU
Participants randomized to TAU received the treatment typically provided for depression at the participating PCCs. This could include scheduled contacts with GPs, nurses and other personnel at the PCC, face-to-face-psychotherapy, antidepressants, sick leave certification and combinations of these treatments depending on the practices and available resources at the specific PCC. However, patients randomized to TAU did not receive ICBT, and ICBT was the only type of psychotherapy delivered to patients randomized to ICBT. Because of the fact that the study was designed as an effectiveness study, there were no other restrictions in additional care offered to patients by the PCCs after inclusion. All patients in the study could receive usual care both before and after the 3-month intervention period, all along special care demands of the individual patient.
Instruments
Diagnostic procedure and patient characteristics
For the diagnostic procedure, we used the following validated instruments: the MINI version 6 and MINI plus, with additions from the sections concerning depression and dysthymia [Citation17], and BDI-II [Citation18]. The MADRS-S [Citation19] was used to exclude patients with severe depression. Questionnaires were used to collect information concerning patient characteristics. The patients answered BDI in conjunction with the assessment, and the rest of the questionnaires at the visit to the research nurse. At 3-month follow-up, the questionnaires were answered at the research nurse visit, and at 6- and 12-month follow-up the questionnaires were sent to the patients.
Primary outcome measures
Depressive symptoms were measured by the validated BDI-II [Citation18], based on the total score in the 0–63 point scale; missing items were not accepted in the analysis. A higher score indicated more depressive symptoms. Severity of depressive symptoms was defined according to the manual [Citation24]. The cut-off indication of major depression was ≥14p in BDI-II. In addition, a diagnosis of depression also required a confirmation in the clinical therapist assessment.
Secondary outcome measures
Quality of life was measured by the validated Quality of Life instrument EQ-5D with British tariff [Citation25] and calculated according to Dolan [Citation26]. A higher score indicated a better condition. Psychological distress was measured by the validated 12-item General Health Questionnaire (GHQ-12) [Citation27], calculated with Likert scoring (0, 1, 2, 3) [Citation28]. A higher score indicated more psychological distress. Information on use of antidepressants (yes/no), sedatives (yes/no), sick leave (yes/no) and number of days of sick leave during 0–3, 4–6 and 7–12 months was collected by questionnaires. The study nurse registered patients’ PCC visits to GPs, therapists and nurses. Visits to GPs and nurses were also collected from electronic patient records (EPR) after the study. Data obtained from the study protocol regarding visits to therapists and nurses and data obtained from EPR regarding GP visits were used in this study.
Statistical methods
The statistical analyses were made using SPSS for Mac, version 20.0. All tests were two-sided. Statistical significance was set at p < 0.05. Standard methods were used for descriptive statistics. Means of intra-individual change were compared between the ICBT group and the TAU group using Student’s t-test. Medication, sick leave and PCC contacts were compared using χ2 test. Days of sick leave were calculated using Mann–Whitney U test. Cohen’s d was calculated for effect sizes for both between groups and within groups.
The Regional Medical Ethics Review Board in Gothenburg, Sweden approved the protocol (Dnr: 696-09, T692-11). Written informed consent was obtained from all participants.
Results
provides an overview of characteristics of the patients in the ICBT and the TAU groups. Except for use of sedatives, there were no significant differences at baseline between patients randomized to ICBT and TAU. Patients lost to follow-up did not differ in baseline characteristics, except that significantly more patients lost to follow-up were living alone.
Table 1. Baseline characteristics for the ICBT group and the TAU group, in total, and for men and women, respectively.
Depressive symptoms (BDI-II)
The intra-individual mean change in scores for depressive symptoms across time periods (0–3, 0–6 and 0–12 months) showed no significant differences between ICBT and TAU. Both patient groups experienced a significant reduction of depressive symptoms during the treatment period, and the positive effect remained at subsequent follow-ups ().
Figure 2. Line charts with error bars of (A) Depressive symptoms (BDI-II), (B) Quality of life (EQ5D) and (C) Psychological distress (GHQ-12), with means before and after treatment and at follow-up at 6 and 12 months for TAU and ICBT, respectively.
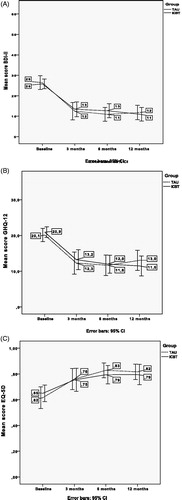
The between group effect sizes (Cohen’s d) differed only marginally (d = 0.09, d = 0.18 and d = 0.09 at 3, 6 and 12 months, respectively). However, the within-group effect sizes were large for both the ICBT group (d = 1.17, d = 1.23 and d = 1.42 at 3, 6 and 12 months, respectively) and the TAU group (d =1.31, d = 1.43 and d = 1.29 at 3, 6 and 12 months, respectively).
Quality of life and psychological distress
Both treatment groups experienced an improved quality of life (). There were no significant differences concerning means of intra-individual change scores between the groups, except for an increased mean intra-individual change from baseline to 6-month follow-up for the TAU group compared to the ICBT group (p = 0.02). This difference leveled out at the 0- to 12-month follow-up. There were no differences between the groups in means of intra-individual change regarding psychological distress in any of the follow-ups; again both groups improved ().
Use of medication
At inclusion, there was no significant difference between the two groups in the proportion using antidepressants. At the 3-month follow-up, however, there were significantly fewer patients in the ICBT group still on antidepressants (). The number of patients using antidepressants increased in the TAU group and decreased in the ICBT group. However, there were no significant differences between the ICBT group and the TAU group concerning antidepressants at the 6- and 12-month follow-ups. Data on antidepressants over the entire observation period showed that 17% in the ICBT group compared to 21% in the TAU group were on antidepressants during the entire treatment period and follow-ups.
Table 2. Use of antidepressants and sedatives at baseline, 3, 6 and 12 months for the patients in the ICBT group and the TAU group, respectively.
Concerning sedatives, there was a significant difference between the groups at baseline; a few patients in the ICBT group used sedatives, compared to none in the TAU group. The difference leveled out at the 3-month follow-up.
Sick leave and PCC contacts
The number of patients on sick leave during the year before inclusion or at 3, 6 or 12-month follow-up did not differ between the groups (). There were no significant differences between the groups concerning number of days on sick leave during 0–3, 4–6 and 7–12 months ().
Figure 3. Proportion (%) and number (n) of patients on sick leave the year before inclusion, during treatment period (0–3 months), and the follow-up periods (4–6 and 7–12 months) in the ICBT group and the TAU group respectively.
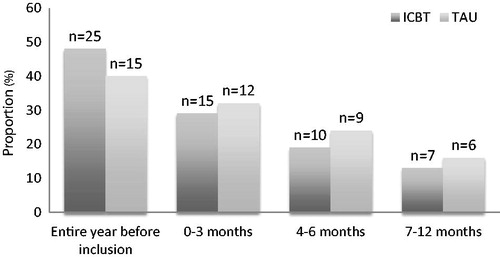
Table 3. Numbers of days of sick leave days 0–3 months, 4–6 months and 7–12 months for the group of patients that were on sick leave for the respective stated period.
Registered visits to PCCs, excluding PRIM-NET activities, showed that the TAU group had significantly more visits to therapists compared to the ICBT group. There was no difference between treatment groups in number of visits to GPs or nurses, or in number of telephone contacts (). The treatment in the TAU group was in 50% antidepressant medication, therapist visits in 39%, regular GP visits in 58% (32% on sick-leave) and in 18% nurse visits ().
Table 4. Number of visits to therapists, GPs and nurses, respectively, and telephone calls to therapists and other personnel for patients in ICBT (n = 52) and TAU (n = 38) during 0–3 months in the PRIM-NET study. There was a significant difference in number of visits to therapist between ICBT and TAU (p = 0.001).
TAU + ICBT patients
To test if main results were changed if the group of 7 patients, initially randomized to TAU, who received ICBT after 3 months (TAU + ICBT) and consequently followed in this study for 12 months only as ICBT patients, we calculated results if TAU + ICBT patients were (i) excluded and (ii) regarded as TAU during 3 months and then excluded, respectively. When the 7 TAU + ICBT patients were excluded, the significant difference between the groups for EQ5D in the 0- to 6-month period disappeared, but on the other hand there was a significant difference in mean intra individual change scores for EQ5-D for 0–12 months (ICBT 11.3, TAU 7.3, p = 0.02). When the 7 TAU + ICBT patients were included as TAU only (for 3 months), there were no differences in the results compared to the presented main results except that there was no significant difference concerning EQ-5D.
The 7 TAU + ICBT patients entered the ICBT treatment with a mean BDI-II score of 19.5 (range = 14–30) compared to 26.7 (range = 8–45) for the patients receiving ICBT or TAU. The patients receiving ICBT after TAU had a intra-individual mean change score for depressive symptoms of −6.2 (SD 7.7) during ICBT treatment period, compared to −12.7 (SD 10.7) for the patients receiving ICBT or TAU.
Discussion
The main results in this randomized controlled study with follow-ups until 1 year after treatment start were that no significant differences in reduction of self-reported depressive symptoms were found between ICBT and TAU, either not directly after treatment or at the 1-year follow-up. Furthermore, there were no differences concerning perception of psychological distress, sick leave frequency or total days of sick leave during the 12-month study period. There was a significant difference concerning antidepressant medication; ICBT patients dropped the medication during the 3-month treatment period, but resumed medication during the following months, and the use of antidepressants was almost the same in both groups at the 6- and 12-month follow-ups. No remarkable differences concerning perception of quality of life could be seen during the 12-month period. Therapist contacts were, as expected, significantly more in the TAU group, but there was no increase in other care contacts with the PCCs during the 3-month treatment period. These findings provide support for ICBT as an equally effective treatment as TAU also in the primary care context for patients accepting ICBT treatment.
Strengths and limitations
The PRIM-NET project was designed as an RCT, targeting depressed patients treated by primary care personnel at PCCs. We employed a rigorous protocol, including diagnostic assessment by trained therapists, an ICBT intervention administered by therapists with CBT training and with experience of working with CBT. Follow-up data were also collected after 6 months and 1 year. We did not exclude any somatic comorbidity. The included patients would therefore represent the typical primary care patients with often several other health problems. We included 90 patients, which is a fairly high number of patients for this type of intervention trial in primary care. The design of the PRIM-NET study enabled assessment of potential treatment outcome differences between ICBT and TAU, and whether ICBT is effective in primary care [Citation14]. Not only depressive symptoms, but other long-term outcomes, important for primary care patients, such as quality of life, medication and sick leave requirements, were included.
The study has a number of limitations. First, despite a prolonged inclusion period and efforts to enhance the enrolment rate, we were not able to include more than 90 patients, which is on the low side of the initial power calculation. The numbers we have for assessed and excluded patients are uncertain. Depression is common in primary care, and we have reason to believe that some patients were deemed not be suitable and never asked about study participation. There is still a risk that there was an outcome difference between TAU and ICBT. However, on the basis of the actual outcomes, with very similar means and variation measures shown in the two groups, the probability of a divergent result when including a 35% higher number of participants is low. The difficulty of meeting estimated inclusion rates in primary care research has been discussed previously [Citation29]. The PCCs in this study were facing several challenges during the time period for the PRIM-NET project. There were large-scale organizational changes and a nation-wide on-going vaccination campaign, to mention a few circumstances that interfered with the research project. The mean age of included patients was 36 years, which is younger than the assumed mean age of all the patients of a typical PCC. Second, trials in primary care often face challenges setting up RCTs with stringency, as it is not possible to set up a blinded trial with a sham group or an untreated control group [Citation15]. In PRIM-NET, our solution was to compare ICBT with TAU. After the first year of a low inclusion rate, despite a large number of patients with depression at the PCCs, an alternative design was set up. To increase the inclusion rate, a centralized unit was formed where all recruited patients were offered ICBT, although for patients randomized to TAU, only after 12 weeks of TAU. In this way, we did not fully follow the randomization scheme, but included 8 ‘extra’ recruited patients to ICBT group who were not included in the present 12-month follow-up study until after being on waiting list for 3 months. Similar procedures have sometimes been applied in long-term ICBT RCTs [Citation30]. Waiting for treatment has its obvious drawbacks, but for practical reasons may sometimes be the next best alternative. The request for psychological treatment often exceeds the actual resources at PCCs, and waiting for psychological treatment is common practice. We have performed all calculations with and without the patients receiving ICBT after 12 weeks of TAU, which shows that the main results remain robust.
Third, regarding diagnosis of depression, there was no therapist assessment after the treatment period, only self-administered protocols. However, many studies on ICBT have used questionnaires of self-rated symptoms of different types, and when diagnostic assessments by trained therapists has been performed it has mostly been only pre-treatment.
Results in relation to other studies
In this study, ICBT was equally effective as TAU. We found no evidence for ICBT being more effective than TAU as found in the trial by Proudfoot et al. [Citation20]. The treatment outcomes in the TAU group in this study were more positive compared to those of the TAU group in Proudfoot et al. [Citation20]. In PRIM-NET, both the ICBT and the TAU groups had a BDI-II score indicating remission of depression (<14p) after treatment. In the Proudfoot et al.’s study [Citation20], only the patients in the intervention group reached a similar reduction in depressive symptomatology. The different outcomes could partially be explained by differences in design. Proudfoot et al. [Citation20] had a study population of patients with depression, anxiety (including phobias and panic) or mixed anxiety and depression, and the computerized CBT was conducted on site at the general practices and with assistance from a nurse. A recent meta-analysis has also shown somewhat reduced effect sizes of CBT in studies over time [Citation31]. The PRIM-NET study was designed to evaluate mild/moderate depression, according to MADRS-S. However, in PRIM-NET, we ended up including also patients with severe depression according to BDI-II. Our results indicated equally good results among severely depressed patients as for those with mild/moderate depression, shown by substantial effect sizes within both the ICBT and the TAU groups in line with the review by Hoifodt et al. [Citation8]. Nevertheless, there are few RCT trials on depression treatment in primary care settings similar to Scandinavian conditions for comparison with our results. Recently, a pragmatic, multicenter RCT study performed in British primary care to assess effectiveness of supported ICBT as an adjunct to usual primary care for adults with depression showed comparable long-term outcomes [Citation31]. Our finding that the reduction of depressive symptoms remained at follow-up is in line also with 8-month follow-up results from a study conducted at general practices in the UK delivering online (synchronous) therapy for depression [Citation12], one study assessing effectiveness of supported ICBT as an adjunct to usual primary care for adults with depression [Citation32], as well as a 3.5-year follow-up study of depressed patients from the general population recruited by advertisement, where 1/3 of the patients initially on waiting list were collapsed into one of the intervention groups (guided self-help ICBT vs. e-mail CBT) [Citation30].
Significance of the study findings
The results of this trial suggests that ICBT with weekly minimal therapist support is non-inferior to the usual treatments in primary care and a treatment alternative also in the long-term perspective for patients with depression in primary care. This knowledge is important for the primary care personnel and the primary care organization, as a majority of patients with depression are treated in primary care.
Notes on contributors
Maria C. M. Eriksson participated in the design of the RCT, was member of the trial management group (TMG), assisted during the whole conduct of the RCT at the PHCC sites, monitored data, drafted and revised the paper.
Marie Kivi participated in the design of the RCT, was member of the trial management group (TMG), assisted during the whole conduct of the RCT at the PHCC sites, monitored data, drafted and revised the paper.
Dominique Hange was member of the TMG, contributed to the trial design, drafted and revised the paper.
Eva-Lisa Petersson was trial manager during the conduct of the RCT, was member of the TMG, drafted and revised the paper.
Nashmil Ariai was member of the TMG, contributed to the trial design, drafted and revised the paper.
Per Häggblad was member of the TMG, contributed to the trial design, drafted and revised the paper.
Hans Ågren was member of the TMG, contributed to the trial design, drafted and revised the paper.
Fredrik Spak was member of the TMG, contributed to the trial design, drafted and revised the paper.
Ulf Lindblad was member of the TMG, contributed to the trial design, drafted and revised the paper.
Boo Johansson was member of the TMG, contributed to the trial design, drafted and revised the paper.
Cecilia Björkelund was chief investigator and initiated the project, designed the RCT, chaired TMG, drafted and revised the paper and is guarantor. All authors had full access to all of the study data and take responsibility for the integrity and accuracy of the data.
Acknowledgements
This work was supported by grants from REHSAM (Swedish Social Insurance Agency) and Region Västra Götaland. The authors wish to acknowledge associate professor Ronny Gunnarsson for contributing with valuable support during planning of the PRIM-NET study.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- World Health Organization. Depression Fact sheet N°369. Geneva: WHO; 2012 [cited 2014 July 28]. Available from: http://www.who.int/mediacentre/factsheets/fs369/en/
- Wells KB, Burnam MA, Rogers W, et al. The course of depression in adult outpatients. Results from the Medical Outcomes Study. Arch Gen Psychiatry. 1992;49:788–794.
- Nieuwenhuijsen K, Faber B, Verbeek JH, et al. Interventions to improve return to work in depressed people. Cochrane Database Syst Rev. 2014;12:CD006237.
- The Swedish National Board of Health and Welfare. National Guidelines for depression and anxiety syndromes. Stockholm 2010 [cited 2014 July 29]. Available from: http://www.socialstyrelsen.se/publikationer2010/2010-3-4
- Andersson G, Cuijpers P. Internet-based and other computerized psychological treatments for adult depression: a meta-analysis. Cogn Behav Ther. 2009;38:196–205.
- Richards D, Richardson T. Computer-based psychological treatments for depression: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32:329–342.
- Cuijpers P, Donker T, van Straten A, et al. Is guided self-help as effective as face-to-face psychotherapy for depression and anxiety disorders? A systematic review and meta-analysis of comparative outcome studies. Psychol Med. 2010;40:1943–1957.
- Hoifodt RS, Strom C, Kolstrup N, et al. Effectiveness of cognitive behavioural therapy in primary health care: a review. Fam Pract. 2011;28:489–504.
- Arnberg FK, Linton SJ, Hultcrantz M, et al. Internet-delivered psychological treatments for mood and anxiety disorders: a systematic review of their efficacy, safety, and cost-effectiveness. PLoS One. 2014;9:e98118.
- Ruwaard J, Schrieken B, Schrijver M, et al. Standardized web-based cognitive behavioural therapy of mild to moderate depression: a randomized controlled trial with a long-term follow-up. Cogn Behav Ther. 2009;38:206–221.
- Andersson G, Hesser H, Veilord A, et al. Randomised controlled non-inferiority trial with 3-year follow-up of internet-delivered versus face-to-face group cognitive behavioural therapy for depression. J Affect Disord. 2013;151:986–994.
- Kessler D, Lewis G, Kaur S, et al. Therapist-delivered internet psychotherapy for depression in primary care: a randomised controlled trial. Lancet 2009;374:628–634.
- de Graaf LE, Gerhards SA, Arntz A, et al. Clinical effectiveness of online computerised cognitive-behavioural therapy without support for depression in primary care: randomised trial. Br J Psychiatry. 2009;195:73–80.
- Roy-Byrne P, Craske MG, Sullivan G, et al. Delivery of evidence-based treatment for multiple anxiety disorders in primary care: a randomized controlled trial. JAMA. 2010;303:1921–1928.
- Andrews G, Cuijpers P, Craske MG, et al. Computer therapy for the anxiety and depressive disorders is effective, acceptable and practical health care: a meta-analysis. PLoS One. 2010;5:e13196.
- Kivi M, Eriksson MC, Hange D, et al. Internet-based therapy for mild to moderate depression in Swedish Primary Care: short term results from the PRIM-NET randomized controlled trial. Cogn Behav Ther. 2014;43:289–298.
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33.
- Arnau R, Meagher MW, Norris MP, et al. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol. 2001;20:112–119.
- Montgomery SA, Asberg M. A new Depression Scale Designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389.
- Proudfoot JRC, Everitt B, Shapiro DA, et al. Clinical efficacy of computerised cognitive-behavioural therapy for anxiety and depression in primary care: randomised controlled trial. Br J Psychiatry. 2004;185:46–54.
- KBTOnline. Depressionshjälpen® [cited March 7, 2017]. Available from: http://www.depressionshjalpen.se/landing/dep/om.html
- Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006;74:658–670.
- Churchill RMT, Furukawa TA, Caldwell DM, et al. ‘Third wave’ cognitive and behavioural therapies versus treatment as usual for depression. Cochrane Database Syst Rev. 2013:CD008705.
- Beck ATSR, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
- Group E. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208.
- Dolan P. Modeling valuations for EuroQol Health States. Med Care. 1997;35:1095–1108.
- Schmitz N, Kruse J, Heckrath C, et al. Diagnosing mental disorders in primary care: the General Health Questionnaire (GHQ) and the Symptom Check List (SCL-90-R) as screening instruments. Soc Psychiatry Psychiatr Epidemiol. 1999;34:360–366.
- Banks M, Clegg CW, Jackson PR, et al. The use of the General Health Questionnaire as an indicator of mental health in occupational studies. J Occup Psychol. 1980;53:187–194.
- Newby JM, Mackenzie A, Williams AD, et al. Internet cognitive behavioural therapy for mixed anxiety and depression: a randomized controlled trial and evidence of effectiveness in primary care. Psychol Med. 2013;43:2635–2648.
- Andersson G, Hesser H, Hummerdal D, et al. A 3.5-year follow-up of Internet-delivered cognitive behavior therapy for major depression. J Ment Health. 2013;22:155–164.
- Johnsen TJ, Friborg O. The effects of cognitive behavioral therapy as an anti-depressive treatment is falling: a meta-analysis. Psychol Bull. 2015;141:747–768.
- Gilbody S, Littlewood E, Hewitt C, et al. Computerised cognitive behaviour therapy (cCBT) as treatment for depression in primary care (REEACT trial): large scale pragmatic randomised controlled trial. BMJ. 2015;351:h5627.
