Abstract
Objective: Elevated calcium concentration is a commonly used measure in screening analyses for primary hyperparathyroidism (pHPT) and cancer. Low bone mineral density (BMD) and osteoporosis are common features of pHPT and strengthen the indication for parathyroidectomy. It is not known whether an elevated calcium concentration could be a marker of low BMD in suspected pHPT patients with a normal parathyroid hormone concentration.
Purpose: To study if low BMD and osteoporosis are more common after ten years in patients with elevated compared with normal calcium concentrations at baseline.
Design: Prospective case control study.
Setting: Primary care, southern Sweden.
Subjects: One hundred twenty-seven patients (28 men) with baseline elevated, and 254 patients (56 men) with baseline normal calcium concentrations, mean age 61 years, were recruited. After ten years, 77% of those still alive (74 with elevated and 154 with normal calcium concentrations at baseline) participated in a dual energy x-ray absorptiometry measurement for BMD assessment and analysis of calcium and parathyroid hormone concentrations.
Main outcome measures: Association between elevated and normal calcium concentration at base-line and BMD at follow-up. Correlation between calcium and parathyroid hormone concentrations and BMD at follow-up.
Results: A larger proportion of the patients with elevated baseline calcium concentrations who participated in the follow-up had osteoporosis (p value = 0.036), compared with the patients with normal concentrations. In contrast, no correlation was found between calcium or parathyroid hormone concentrations and BMD at follow-up.
Conclusions: In this study, patients with elevated calcium concentrations at baseline had osteoporosis ten years later more often than controls (45% vs. 29%), which highlights the importance of examining these patients further using absorptiometry, even when their parathyroid hormone level is normal.
Osteoporosis is common, difficult to detect and usually untreated. It is not known whether elevated calcium concentrations, irrespective of the PTH level, could be a marker of low bone mineral density.
No correlation was found between calcium or parathyroid hormone concentrations and bone mineral density at follow-up.
In this study, patients with elevated calcium concentrations at baseline had osteoporosis ten years later more often than controls (45% vs. 29%).
Key Points
Introduction
An elevated calcium concentration in serum and plasma is a marker of a multitude of illnesses, the most prevalent being primary hyperparathyroidism (pHPT), cancer, renal disease and sarcoidosis [Citation1]. The possibilities offered by multichannel biochemical analysis, introduced some decades ago, have led to increased use of the calcium analysis in primary care [Citation2–4] in screening for pHPT, a condition difficult to detect due to the often subtle symptoms [Citation5,Citation6]. Among other things, pHPT causes secondary osteoporosis, with low bone mineral density (BMD) [Citation7,Citation8], also in mild or asymptomatic pHPT [Citation5,Citation8,Citation9]. Not all patients with pHPT are treated surgically, but the presence of osteoporosis strengthens the indication for parathyroidectomy [Citation10]. Measurement of BMD is therefore included in the investigation of patients with suspected pHPT [Citation9].
Osteoporosis is very common in Sweden [Citation11,Citation12] and is difficult to detect, as there are few symptoms before a fracture occurs [Citation13,Citation14]. For this reason, it is often undertreated [Citation15]. The first clinical manifestations of osteoporosis are fragility fractures, primarily of the distal forearm, the humerus or the proximal femur, often caused by minor trauma [Citation16], or of the vertebrae, without apparent trauma, which make them difficult to detect [Citation17]. Factors known to increase the risk of fragility fractures are smoking, family history of hip fractures, oral corticosteroid medication, body mass index below 20 kg/m2, extensive alcohol intake and menopause before age 45 years [Citation12,Citation16].
Whether elevated calcium concentrations are associated with an increased risk of osteoporosis is controversial. In a population-based screening study, serum calcium was negatively correlated with BMD in both the vertebrae and the total body. However, parathyroid hormone (PTH) had no association with BMD at any site [Citation18]. Other studies, on the other hand, found no relationship between BMD and calcium [Citation19,Citation20] or PTH concentrations [Citation19].
In primary health care, many patients with single elevated calcium concentrations do not have pHPT [Citation21]. Still, if there is a relationship between calcium concentrations and BMD or osteoporosis it would indicate a need to investigate patients with elevated calcium concentrations further, despite normal PTH levels, with dual energy x-ray absorptiometry (DXA). Efforts to find patients with osteoporosis before they are affected by complications are essential to reduce suffering and death for the individual patient and increased costs for society [Citation12,Citation22].
The primary aim of this study was to investigate if osteoporosis at follow-up is more common among patients with elevated or normal calcium concentrations at baseline in the primary health care setting. The secondary aim was to investigate if there is a correlation between baseline calcium and PTH concentrations or BMD at follow-up.
Material and methods
Study subjects
Tibro is a rural community in Sweden with 11,000 inhabitants and with only one primary health care centre (HCC) during the study period. The medical records of all patients with elevated calcium concentrations between 1995 and 2000 (baseline) have previously been studied [Citation23], . At baseline, 127 patients with an elevated calcium concentration ≥2.56 mmol/l (reference range 2.15–2.55) in at least one test were identified. Two age and sex-matched controls (n = 254) from the same HCC with baseline calcium <2.45 mmol/l were selected for each patient, as previously described [Citation6,Citation21]. Individuals with baseline calcium concentrations between 2.45–2.55 mmol/L were excluded to get a better contrast between the groups. Diseased participants (41%, 53/127 with baseline elevated calcium; 39%, 100/254 with normal calcium concentrations) and those not investigated with DXA (5%, 6/127 with elevated, and 6%, 14/254, with normal calcium concentrations at baseline respectively) were omitted from the current study.
Figure 1. Flow chart of study subjects, including drop-outs, with elevated and normal calcium concentrations at base line at Tibro Health Care Centre, Sweden: first investigation in 1995–2000, and re-examination in 2009–2010.
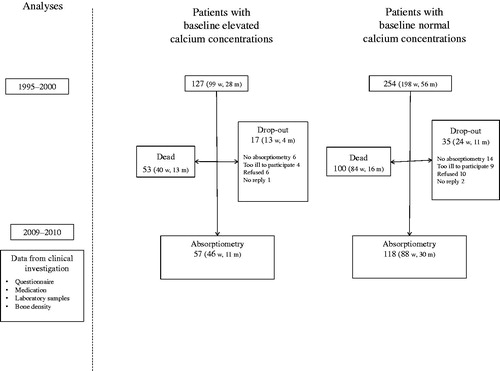
All study participants who were alive at the time of follow-up in July, 2011; 74 (127–53, 58%) with elevated and 154 (254–100, 61%) with normal calcium concentrations at baseline were invited by mail to participate. Non-responders were contacted by telephone. At a visit to the HCC, all participants were interviewed by a nurse about their medical history and current medication using a structured questionnaire. Non-fasting blood samples were drawn and analysed for calcium (reference range 2.15–2.50), ionised calcium and intact PTH [Citation6, Citation21]. Individuals who had moved from the community were interviewed by phone, and blood samples were taken at their local HCC. The study was performed according to the declaration of Helsinki and approved by the ethics committee of the Regional Ethical Review Board in Gothenburg, 2008 (Reg. no. 696-07).
Bone density measurement
BMD was measured by DXA at Unilabs in Skövde, using the Lunar Prodigy bone densitometer (GE Medical Systems Lunar Corporation, Madison, Wisconsin, USA). All measurements were made with the same densitometer, except in patients (n = 10) who had moved to other parts of the country. In these patients, the BMD measurement was carried out using eight other densitometers from the same manufacturer. BMD is reduced in pHPT patients, especially in cortical bone, such as the radius [Citation7,Citation24]. We therefore supplemented the accepted osteoporosis diagnosis method according to the WHO with measurement of BMD in the lumbar spine (L2 to L4) in the anterior-posterior direction and the femur neck, using DXA [Citation16] with the non-dominant radius.
The results are presented as BMD (g/cm2) and a T-score. T-scores were calculated as the difference in standard deviations (SD) from the mean for the patient’s age and sex. The patients in the study were Caucasian with few exceptions, and non-Hispanic whites were used as the reference population (provided by the manufacturer). The + values indicate higher and the − values lower BMD on DXA, compared with the reference population. According to the WHO definition, a T-score of −1.0 or higher is normal, a T-score of −2.5 or lower indicates osteoporosis, and osteopaenia is defined as a T-score between −2.5 and −1.0 [Citation16].
Osteoporosis and osteopaenia
We divided the participants into three groups based on the results of the BMD measurements at follow-up: osteoporosis, osteopaenia and normal BMD. If discordant findings were seen in different skeletal locations in a patient, the lowest value defined the group to which the patient belonged. A lateral projection with vertebral fracture assessment was performed in connection with the BMD measurement at follow-up in order to detect vertebral compression fractures, and was assessed at the discretion of the DXA physician and the co-author (EA) [Citation17].
Statistics
Descriptive statistics were presented and comparisons between groups were performed using the T test, the Chi-squared test and the Mann-Whitney U test, depending of the type of data included in the analysis. Data are given as means ± SD and medians with the 10th and 90th percentile in , and as means ± SD in . The association between two variables was tested using the Spearman rank correlation test. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS 22.0 statistical package for Windows (Armonk, NY: IBM Corp).
Table 1. Characteristics of patients with elevated and normal calcium concentrations at baseline, 1995–2000 and 2008–2010, men and women, at Tibro Health Care Centre, Sweden.
Table 2. Clinical characteristics and bone mineral variables, 2009–2010, in patients with elevated and normal calcium concentrations at baseline, 1995–2000 and 2008–2010, men and women, at Tibro Health Care Centre, Sweden.
Results
Of the patients still alive at follow–up, 74 (15 men) had elevated and 154 (40 men) had normal calcium concentrations at baseline, . The participation rate in the DXA measurement in those still alive was the same in the group with elevated as in the group with normal calcium concentrations at baseline, 77%. The most common reasons for not attending the DXA assessment were being bed-ridden or having severe dementia. However, due to more deaths among men with baseline elevated calcium concentrations, BMD was measured at follow-up in 11/28 (39%) men in the elevated calcium group vs. 30/56 (52%) in the normal calcium group.
Study subjects
The characteristics of the study subjects are presented in . In the group with baseline elevated calcium concentrations, the median value at baseline was 2.60 (2.56–2.78, 10th and 90th percentile), and 2.45 mmol/L (2.27–2.58, 10th and 90th percentile) at follow-up. Body constitution (body height and weight) did not differ between the groups with elevated and normal calcium concentrations at baseline. Furthermore, the only significant difference between two groups was that the total group and women with baseline elevated calcium concentrations had been using oral corticosteroids for long periods compared with those with normal calcium concentrations. Thus there no significant differences between the groups with elevated and normal calcium concentrations at baseline regarding previous fractures (distal forearm, humerus, proximal femur and vertebra), family history of fractured hip, age at menopause, smoking habits or low BMI, and medication with multivitamins, oestrogens or dihydrotachysterol (data not shown).
Bone density measurement
Women with baseline elevated calcium had lower BMD at follow-up than, the group with baseline normal calcium concentrations; however, the difference was not significant, . No correlation was found between serum calcium or PTH and BMD at follow-up (data not shown).
Osteoporosis and osteopaenia
The majority of the patients who participated in the follow-up were diagnosed with osteoporosis or osteopaenia, . In the group with baseline elevated calcium concentrations, there was a greater proportion of patients with osteoporosis, 45% vs. 29%, but a smaller proportion of patients with osteopaenia, 29% vs. 49%, compared with the group with normal calcium concentrations (p value = 0.036). As expected, osteoporosis was more common among women than men, . More than 50% of the women with baseline elevated calcium concentrations had osteoporosis.
Figure 2. Patients with elevated and normal calcium concentrations divided at baseline into three groups, depending on the results of the absorptiometry: normal bone density ≥ −1 SD, osteopaenia: < −1.0 SD and > −2.5 SD, osteoporosis: ≤ −2.5 SD. Re-examination in 2009–2010 of patients from Tibro Health Care Centre, Sweden. Panel A. All patients. Panel B. Patients divided by gender.
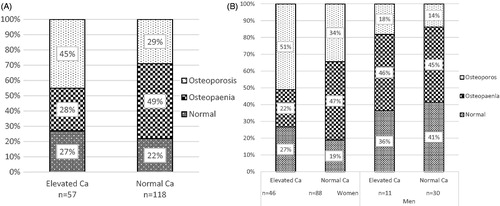
Vertebral fracture assessment was performed in approximately 90% of the bone density measurements. Fifteen per cent of the patients with elevated (9/57) and 14% with normal calcium concentrations (17/118) at baseline had vertebral compression fractures (p value = 0.83), . The majority of the patients with vertebral fractures met the criteria for a diagnosis of osteoporosis by DXA, but four patients with elevated and three with normal calcium concentrations at baseline had osteopaenia. More than50% in the total group with baseline elevated calcium concentrations had osteopaenia combined with manifest vertebral fractures, or osteoporosis (data not shown).
Discussion
In this reinvestigation, significantly more patients with baseline elevated calcium concentrations had osteoporosis, compared with controls with baseline normal calcium concentrations (45% vs. 29%). This difference remained, despite treatment of patients with pHPT according to clinical guidelines [Citation9,Citation10] and ten years having lapsed since the baseline investigation.
Bone density measurement
Some studies found a correlation between calcium and BMD [Citation18], while others did not [Citation19,Citation20]. In our study we could not find a correlation between baseline calcium and BMD ten years later. One explanation could be that the BMD measurement was performed in a follow-up survey, in which the majority of those with baseline elevated calcium concentrations had been normalised, compared with other studies with higher calcium concentrations in connection with the BMD investigation [Citation18].
Osteoporosis and osteopaenia
We could not find any correlation between the baseline calcium concentration and the follow-up BMD levels. However, there was an association between the calcium concentrations when patients were assigned to the clinical conditions of osteoporosis, osteopaenia and normal BMD based on the overall BMD assessment. Because the material was small, the pathological outcomes in the different measurement localisations were few and no difference could be detected when these points were studied separately or when osteoporosis, osteopaenia and normal BMD were distributed by gender. On the other hand, the overall assessment and assignment of patients to the diagnoses of osteoporosis and osteopaenia are clinically relevant and the rationale for treatment [Citation12]. Osteoporosis in 29% of patients with normal calcium concentrations is in line with the report by the Swedish National Board of Health and Welfare, which shows that osteoporosis diagnosed by BMD is present in every third woman aged 70–79 years [Citation12].
Vertebrae affected by degenerative changes with reactive osteophytes may result in a misleading, high BMD measurement, and the diagnosis of osteoporosis may thus be underestimated [Citation25]. This is most common among elderly women, of whom there was a large number in our study. Under these conditions, it is a great advantage to include a vertebral fracture assessment and a comprehensive assessment in the investigation. Vertebral compression fractures — another obstacle in the assessment — may also be found, which is important, as these patients should receive prophylactic treatment for osteoporosis, irrespective of their BMD when over 50 years of age [Citation17].
The group with baseline elevated calcium concentrations had been treated with oral corticosteroids for extended periods more frequently than the group with normal calcium concentrations. Corticosteroids have a wide variety of effects on bone, largely due to a reduction in bone formation combined with sustained bone destruction [Citation26]. Fractures are one of the most feared side effects. It is, however, less likely that corticosteroids are the cause of the elevated calcium concentrations, as they decrease the intestinal calcium absorption by decreasing the expression of calcium channels in the duodenum [Citation27]. Nowadays, bisphosphonate should be given to patients at risk at the start of treatment with an expected duration >3 months [Citation12, Citation28]. Unfortunately, we have no data on bisphosphonate treatment in patients on oral corticosteroids for long periods of time. A blueprint for future research would be to study the potential association between elevated calcium concentrations and oral glucocorticoid treatment.
There has been increasing interest around the world in recent years in a variant of pHPT that seems to be little known in primary care; a mild type of pHPT where the patient has an elevated calcium concentration with a normal, non-suppressed PTH concentration [Citation4]. The normal physiological reaction to elevated calcium should be a very low or undetectable PTH concentration [Citation10,Citation29]. Measurement of BMD in patients with elevated calcium concentrations and normal PTH concentrations, who do not seem to be candidates for parathyroidectomy, could lead to an early diagnosis of osteoporosis before fractures develop. By using 24-hour urine calcium sampling is it possible to distinguish these patients from patients with the unusual, congenital and benign condition of familial hypercalcaemic hypocalciuria, which is not ameliorated by surgery [Citation10].
Strength
The strength of this study was the high participation rate, 77%, in the DXA investigation, probably due to the study being confined to one HCC and transport to the survey site being offered to those who so desired. Another advantage was that the study setting in ordinary primary health care, which is of benefits when inferring the results to other primary health care settings [Citation30]. To our knowledge, no previously published study has addressed hypercalcaemia irrespective of PTH levels and its association with BMD in the primary health care setting.
Limitation
In this follow-up, 42% of patients with elevated and 39% with normal calcium concentration at baseline were diseased at follow-up and could not participate. Moreover, those who participated had been treated during follow-up, for example with bisphosphonates, which may have increased their BMD at follow-up [Citation8,Citation12]. Furthermore, almost a fourth of the group with baseline elevated calcium concentrations had undergone parathyroidectomy, which may have increased their BMD after ten years [Citation18]. DXA investigations at baseline would have been a better option according to the objective of the study. In the current study, the effect of the markers at baseline was probably diluted as a result of different treatment effects during follow-up.
Furthermore, for practical reasons the DXA examinations were carried out using several different devices that were not calibrated against each other. To minimise deviations in the results, all investigations were performed using devices from the same manufacturer. Analysis of total calcium is used as a screening method in primary health care, but it is subject to many sources of error and should be supplemented with analysis of ionised calcium to determine hypercalcaemia accurately [Citation1]. A prospective study with repeated calcium analyses supplemented by an ionised calcium analysis would have been preferable.
Conclusions
This study showed that patients with baseline elevated calcium concentrations at follow-up had osteoporosis significantly more than controls with baseline normal calcium concentrations (45% vs. 29%). More than 50% of the women with baseline elevated calcium concentrations fulfilled the criteria for osteoporosis. An elevated calcium concentration in a patient should therefore lead to further investigation with BMD measurement by DXA, regardless of the PTH concentration. In a further perspective, these results are of great interest to physicians in order to improve prevention and treatment for patients with osteoporosis, as osteoporosis, low energy fractures and hypercalcaemia are severe conditions involving high costs for society in countries in Northern Europe.
Notes on contributors
Sofia Dalemo is a GP and PhD. Her main area of research is endocrinology. She is a teacher in primary care.
Robert Eggertsen is a GP and Professor at Sahlgrenska Academy, University of Gothenburg. He has a broad competence in a variety of research areas and methods in primary care, with mayor focus on thyroid, hypertension, and endocrinology.
Per Hjerpe is a GP and PhD. His main area of research is data from primary care computerized patient records for quality monitoring and research purposes and participates in the development work of the Regional Quality Database for Primary Care in Gothenburg.
Erik G Almqvist is a specialist in Endocrinology and PhD. His previous research has focused on endocrinology and bone mineral density.
Kristina Bengtsson Boström is a GP and Associate Professor at University of Lund. She has a broad competence in a variety of research areas and methods in primary care, her main areas of research are hypertension. She is an experienced teacher in primary care.
Acknowledgements
The study was financed by The Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland; the Skaraborg Research and Development Council, the Skaraborg Primary Care Research and Development Council, the Skaraborg Institute, Skövde, and The Swedish Society of Medicine. We cordially thank the staff at Tibro Health Care Centre for help with the data collection, and Johanna Låstberg for her help with preparing the manuscript.
Disclosure statement
The authors report no conflicts of interest.
References
- Endres DB. Investigation of hypercalcemia. Clin Biochem. 2012;45:954–963.
- Nordenstrom E, Katzman P, Bergenfelz A. Biochemical diagnosis of primary hyperparathyroidism: Analysis of the sensitivity of total and ionized calcium in combination with PTH. Clin Biochem. 2011;44:849–852.
- Dalemo S, Hjerpe P, Ohlsson H, et al. Variation in plasma calcium analysis in primary care in Sweden – a multilevel analysis. BMC Fam Pract. 2010;11:43.
- Lindstedt G, Nystrom E, Lundberg PA, et al. Screening of an elderly population in primary care for primary hyperparathyroidism. Scand J Prim Health Care. 1992;10:192–197.
- Applewhite MK, Schneider DF. Mild primary hyperparathyroidism: a literature review. Oncologist. 2014;19:919–929.
- Dalemo S, Eggertsen R, Hjerpe P, et al. Quality of life and health care consumption in primary care patients with elevated serum calcium concentrations in-a prospective, case control, study. BMC Fam Pract. 2014;15:84.
- Khosla S, Melton LJ, 3rd, Wermers RA, et al. Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res. 1999;14:1700–1707.
- Sankaran S, Gamble G, Bolland M, et al. Skeletal effects of interventions in mild primary hyperparathyroidism: a meta-analysis. J Clin Endocrinol Metab. 2010;95:1653–1662.
- Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3561–3569.
- Udelsman R, Akerstrom G, Biagini C, et al. The surgical management of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3595–3606.
- Svedbom A, Hernlund E, Ivergard M, et al. Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos. 2013;8:137.
- Swedish National Guidelines for Musculoskeletal Diseases. The National Board of Health and Welfare. 2012, up-date 2014. Available from: www.socialstyrelsen.se (last accessed at 15 May 2017).
- Looker AC, Orwoll ES, Johnston CC, Jr., et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761–1768.
- Blivik J, Karlsson MK, Moller M. Screening for low bone mineral density with quantitative ultrasound within the primary health care system. Scand J Prim Health Care. 2004;22:78–82.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25:2359–2381.
- Sozen T, Ozisik L, Basaran NC. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4:46–56.
- Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone. 2017;104:54–65.
- Hagstrom E, Lundgren E, Mallmin H, et al. Positive effect of parathyroidectomy on bone mineral density in mild asymptomatic primary hyperparathyroidism. J Intern Med. 2006;259:191–198.
- Almqvist EG, Becker C, Bondeson AG, et al. Early parathyroidectomy increases bone mineral density in patients with mild primary hyperparathyroidism: a prospective and randomized study. Surgery. 2004;136:1281–1288.
- Sutlovic D, Boric I, Sliskovic L, et al. Bone mineral density of skeletal remains: discordant results between chemical analysis and DXA method. Leg Med (Tokyo). 2016;20:18–22.
- Dalemo S, Eggertsen R, Hjerpe P, et al. Long-term follow-up of patients with elevated serum calcium concentrations in Swedish primary care. Scand J Prim Health Care. 2013;31:248–254.
- Stam H, Harting T, van der Sluijs M, et al. Usual care and management of fall risk increasing drugs in older dizzy patients in Dutch. general practice. Scand J Prim Health Care. 2016;34:165–171.
- Dalemo S, Hjerpe P, Bostrom Bengtsson K. Diagnosis of patients with raised serum calcium level in primary care, Sweden. Scand J Prim Health Care. 2006;24:160–165.
- Adami S, Braga V, Squaranti R, et al. Bone measurements in asymptomatic primary hyperparathyroidism. Bone. 1998;22:565–570.
- SBU. Mätning av bentäthet (Measurement of bone mineral density). In: Swedish Agency for Health Technology Assessment and Assessment of Social Services, SBU-rapport 127; 1995.
- Henneicke H, Gasparini SJ, Brennan-Speranza TC, et al. Glucocorticoids and bone: local effects and systemic implications. Trends Endocrinol Metab. 2014;25:197–211.
- Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328.
- Panday K, Gona A, Humphrey MB. Medication-induced osteoporosis: screening and treatment strategies. Therapeut Adv Musculoskeletal Dis. 2014;6:185–202.
- Chen H. Primary Hyperparathyroidism. In: Cameron AM, editors. Current surgical therapy. Philadelphia (PA): Elsevier Saunders; 2011. p. 787–792.
- Thörneby A, Nordeman LM, Hellebö Johanson E, No association between level of vitamin D and chronic low back pain in Swedish primary care: a cross-sectional case-control study. Scand J Prim Health Care. 2016;34:196–204.
