Abstract
Objective
The aim was to compare rapid antigen detection test (RADT) and throat culture for group A streptococci (GAS) among patients recently treated with penicillin V for GAS pharyngotonsillitis.
Design and setting
The study was a secondary analysis within a randomized controlled trial comparing 5 versus 10 days of penicillin V for GAS pharyngotonsillitis. Patients were recruited at 17 primary health care centres in Sweden.
Subjects
We included 316 patients ≥ 6 years of age, having 3–4 Centor criteria, a positive RADT and a positive throat culture for GAS at inclusion, and also having a RADT and throat culture for GAS taken at a follow-up visit within 21 days.
Main outcome measures
RADT and conventional throat culture for GAS.
Results
This prospective study showed 91% agreement between RADT and culture at follow-up within 21 days. Only 3/316 participants had negative RADT with a positive throat culture for GAS at follow-up, and 27/316 patients with positive RADT had a negative culture for GAS. Log rank test did not reveal any difference in the decline over time of positive tests between RADT and throat culture (p = 0.24). Agreement between RADT and throat culture for GAS at the follow-up was not associated with treatment duration, number of days from inclusion until follow-up, throat symptoms at follow-up, gender, or age.
Conclusion
RADT and culture for GAS agreed to a high extent also after recent penicillin V treatment. RADT for GAS means a low risk for missing the presence of GAS.
Testing for group A streptococci (GAS) before antibiotic treatment can reduce antibiotic prescription for pharyngotonsillitis. It has been proposed that rapid antigen detection tests (RADT) for group A streptococci after recent penicillin V treatment may be falsely positive due to possible persisting antigens from non-viable bacteria.
The decline of the presence of GAS was similar between RADT and conventional throat culture in patients who had recently completed penicillin V treatment for GAS pharyngotonsillitis
RADT for GAS is useful in identifying the presence of GAS after recent penicillin V treatment
KEY POINTS
Introduction
Group A streptococci (GAS) pharyngotonsillitis is a common cause for antibiotic prescription in primary care [Citation1–3], and penicillin V is the recommended treatment in several countries [Citation4–7]. Due to the emergence of antimicrobial resistance, it is important to restrict the use of antibiotics [Citation8,Citation9].
Antibiotic treatment for GAS pharyngotonsillitis was introduced in the late 1940s in a totally different epidemiological context. Back then severe complications, such as rheumatic fever, were far more common [Citation10]. Today such complications are seen mainly in low-income countries and in first nation people in some high-income countries [Citation11]. Since acute uncomplicated GAS pharyngotonsillitis remits spontaneously [Citation12], the main reason for antibiotic treatment in high-income countries is to accelerate the resolution of symptoms [Citation4,Citation13]. This modest benefit from treatment is seen in patients with the confirmed presence of GAS [Citation12].
The clinical diagnosis of sore throat is a challenge. Signs and symptoms cannot distinguish between different bacterial or viral causes of sore throat [Citation14,Citation15]. Using absence of GAS as a stopping rule may reduce antibiotic prescription for pharyngotonsillitis in both adults and children [Citation16–18].
A major advantage of a rapid antigen detection test (RADT) for GAS is that it is a point-of-care test, and quick to perform [Citation19,Citation20]. However, the usefulness of RADT has been questioned because of its limited and variable sensitivity when compared with conventional culture techniques [Citation21]. Nonetheless, the sensitivity of RADT for GAS is equal to the sensitivity of conventional throat culture when the RADT is compared to more advanced culture techniques [Citation22] or PCR [Citation23].
Approximately 10% of patients experience treatment failure or early relapse. When these patients come back the question is if the appropriate test is a RADT or conventional culture. The usefulness of RADT after recent antibiotic treatment of GAS pharyngotonsillitis has been specifically questioned since RADT requires only the presence of antigens to be positive. The concern was for antigens from non-viable bacteria to persist after antibiotic treatment, and thus result in false positive RADT for GAS [Citation24,Citation25].
There are few previous studies of RADT after antibiotic treatment for GAS pharyngotonsillitis [Citation24–26]. These studies, which used an older type of RADT (latex agglutination technique), were very small or had a design that did not clarify if RADT for GAS was useful after recent penicillin V treatment for pharyngotonsillitis.
The primary aim of this study was to compare the outcome of a modern immunoassay RADT for GAS and conventional throat culture for GAS among patients with a recent GAS pharyngotonsillitis treated with penicillin V.
Material and methods
This was a secondary analysis of a randomised controlled trial of penicillin V treatment of 5 versus 10 days in patients with pharyngotonsillitis and confirmed presence of GAS [Citation27]. The participants were recruited from 17 primary healthcare centres in Sweden between September 2015 and February 2018. All participating patients and their eventual guardians were informed of the study, verbally and in writing. All included patients or their guardians provided written consent prior to participation.
Participants
The main study recruited 433 patients ≥6 years of age attending primary care for sore throat having 3 or 4 Centor criteria and a positive RADT for GAS. Participants were randomized to penicillin V 800 mg four times daily for 5 days or 1,000 mg three times daily for 10 days. 422 patients received the allocated penicillin V treatment. The main study had a number of exclusion criteria related to serious illness, immunomodulating treatment and recent antibiotic courses [Citation27]. All 422 patients had positive RADT for GAS, however, not all had a positive throat culture for GAS at inclusion. In this secondary analysis we only used data from patients who received the allocated treatment and 1) had a positive RADT for GAS and a positive throat culture for GAS at inclusion, and 2) also had a conclusive RADT and throat culture at a follow-up within 21 days from inclusion. Consequently, all patients in this secondary analysis had both positive RADT and positive culture for GAS from start, which enabled a comparison of the decline in these two positive test results, respectively.
Data collection
At inclusion all patients were clinically examined by a physician. A case report form was completed covering the Centor criteria, clinical signs, age, gender and the allocated treatment regimen (5 or 10 days). Throat swabs for RADT and culture were performed at the inclusion visit. To reduce discomfort for children a double swab method was used, rotating the two swabs towards the tonsils and pharynx at one time. All patients were offered a single follow-up visit with a physician within 5–7 days after completion of the antibiotic treatment. Due to preferences from participants some follow-up visits took place earlier or later on than 5–7 days after completed antibiotic treatment. The follow-up visit included an examination by a physician evaluating clinical cure, and throat swabs were obtained for RADT and culture for GAS.
The RADT was performed according to instructions from the manufacturer with the locally available equipment. The tests used were either a OSOM Strep A test manufactured by Sekisui Diagnostics, or a QuickVue DipStick Strep A test manufactured by Quidel. Both are RADT immunoassay tests for GAS. Swabs for throat culture were sent to regional microbiological laboratories for culture on blood agar plates incubated overnight at 35–37 °C.
Statistical analysis
Descriptive statistics were compiled including a cross-tabulation of the outcome of RADT for GAS and throat culture for GAS at the follow-up visit. Kaplan Meier curves were used as a visual presentation of the proportion of RADT and throat cultures positive for GAS at the single follow-up in each participant within 21 days after inclusion. A log-rank test was performed to compare the distributions of positive RADT and culture for GAS at these follow-up visits.
A multivariable binary logistic regression analysis was used to evaluate possible factors associated with agreement between RADT and throat culture for GAS at the follow-ups. Agreement at the follow-up visit was used as the dependent variable. The independent variables were: treatment regimen (5 or 10 days), number of days from inclusion, clinical cure at follow-up (yes/no), gender and age (children 6–15 years versus 16 years and older). IBM SPSS statistics version 27 was used for statistical analysis and the level of significance was set to 0.05.
Results
Study population
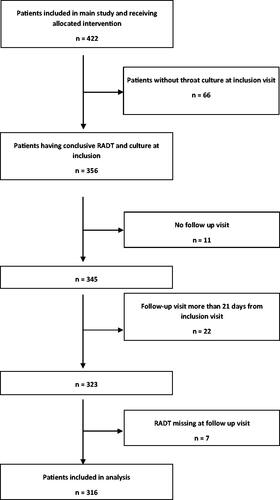
Of the 422 patients in the main study, we excluded 66 without a positive throat culture for GAS at inclusion, 33 who were not evaluated at a follow-up visit within 21 days from inclusion and seven where RADT for GAS was not performed at the follow-up. Hence, 316 patients were eligible and included in this secondary analysis (). Among the participants 66% (210/316) were women and 34% (106/316) men. The median age was 31 and the mean age 29 years (SD 14, IQR 17–38), and 24% (77/316) were children aged 6–15 years. Fifty-six percent (176/316) received the five-day treatment regimen and 44% (140/316) the 10-day. The 316 participants were followed-up between 6–21 days (median 13 days) from inclusion and 9.5% (30/316) of them reported some level of remaining symptoms at the follow-up.
RADT and throat culture findings at follow-up
The proportion of positive RADTs for GAS was 21% (66/316) and the proportion of positive throat cultures for GAS was 13% (42/316) at the follow-up visits 6–21 days after inclusion (). The test results from RADT and throat culture for GAS at follow-up were in agreement in 91% (286/316) of the participants. Neither of the three participants with negative RADT and a positive culture had abundant growth in the culture nor symptoms of a sore throat (). In comparison, most of the patients with positive cultures at follow-up had an abundant growth of GAS.
Table 1. Rapid antigen detection test (RADT) and throat culture for group A streptococci (GAS) at follow-up visit.
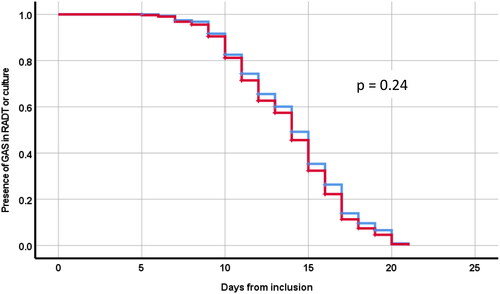
The Kaplan Meier curves visualize the proportion of RADT and throat cultures positive for GAS at each day after inclusion (day 6–21) (). The log rank test showed no significant difference in the decline over time of positive test results between RADT and throat culture at these follow-ups (p = 0.24) ().
Figure 2. Kaplan Meyer curves illustrating proportion of GAS positivity in RADT (blue curve) and throat culture (red curve) at a follow-up visit within 21 days from inclusion.
The multivariable logistic regression found no correlation between agreement between RADT and throat culture for GAS at the follow-up visit and any of the independent variables: five days’ treatment OR 0.90 (0.34–2.4; p = 0.83), number of days from inclusion until follow-up OR 1.0 (0.89–1.2; p = 0.64), throat symptoms at follow-up OR 1.1 (0.30–3.8; p = 0.92), male gender OR 0.81 (0.36–1.94; p = 0.62) and age ≥ 15 years OR 0.53 (0.24–1.2; p = 0.12).
Discussion
This prospective study showed 91% agreement between RADT and culture at follow-up within 21 days. Negative RADT with a positive throat culture for GAS was very rare at follow-up within 21 days from start of penicillin V treatment for pharyngotonsillitis. At follow-up, 27 patients had positive RADT and negative throat culture for GAS. A log-rank test revealed no differences in the decline over time of positive tests between RADT and throat culture. Agreement between RADT and throat culture for GAS at the follow-up visit was not associated with treatment duration, number of days from inclusion, throat symptoms at follow-up, gender, or age.
Strengths and weaknesses of the study
This study focused solely on evaluating the possibility of a false positive RADT due to non-viable remains of GAS after antibiotic treatment of pharyngotonsillitis. It should be kept in mind that this study did not focus on management of patients with recurrent tonsillitis, which is another research topic.
In our study women constituted approximately 2/3 of the study patients. Though females generally report more sore throat symptoms than males [Citation2] there are probably additional causes for the female overrepresentation among participants. However, the statistical analysis gave no indication of gender influencing the results.
A limitation of this study is that each patient had only one follow-up visit. Daily follow-up testing could have provided more information on diagnostic tests and presence of GAS after treatment. Another limitation of the study was the lack of a PCR test for GAS.
Findings in relation to other studies
Negative RADT when culture is positive
We found that negative RADT for GAS with positive throat culture was very rare at follow-up. None of the three patients with negative RADT and a positive culture had abundant growth of GAS in the throat culture, and none had throat symptoms. This is in line with earlier studies on RADT sensitivity, compared to throat culture for GAS, which found the RADT sensitivity to be lower in patients with mild growth of GAS compared to patients with abundant growth of GAS in the throat culture [Citation28–30].
Unlike us, Sheeler et al. studied results from RADT and standard throat culture for GAS in patients with relapse of symptoms within 28 days from prior antibiotic treatment for streptococcal pharyngitis [Citation24]. They found 10% (10/104) negative RADTs among the symptomatic patients with positive culture for GAS after treatment. In their control group consisting of patients with new pharyngotonsillitis they found 30% (19/63) negative RADTs among patients with positive culture. The surprisingly disparate results could be related to different patient management: the relapse patients were assessed by physicians and the controls by nurses. In comparison we found 7% (3/42) negative RADTs among patients with positive throat culture for GAS.
Scheeler et al. recommended back-up throat cultures when RADT for GAS was negative. Since we found few negative RADTs with a positive culture for GAS, we conclude that using RADT implied a low risk for missing presence of GAS. In contrast to Sheeler et al. we see no reason for a back-up culture when RADT for GAS is negative.
Positive RADT when culture is negative
We found 27 patients having positive RADT and negative throat culture for GAS at follow-up.
An earlier study compared RADT for GAS to a combination of routine throat culture and additional throat culture media, and found that RADT had higher sensitivity than the routine throat culture [Citation22]. Throat culture has been questioned as the gold or criterion standard for the presence of GAS in the throat [Citation22,Citation23]. We do not know which test method is closest to the ground truth when diagnosing GAS pharyngotonsillitis. Therefore, we chose not to calculate sensitivity and specificity for neither RADT nor culture.
Positive RADTs with negative throat culture for GAS have been associated with abundant contamination of Staphylococcus aureus, suggesting co-colonization of S. aureus to have an inhibitory impact on GAS culture [Citation23]. In addition, positive RADT with negative throat culture for GAS was found in GAS pharyngotonsillitis with strains of GAS without beta-haemolysis. In conventional throat cultures on blood agar plates GAS is detected due to haemolysis. Therefore, non-haemolytic streptococci might not be detected in conventional throat cultures [Citation31].
The similar decline in the proportion of RADT and throat cultures positive for GAS at each day after inclusion () suggests that possible remaining antigen from non-viable GAS does not influence the test outcome.
If diagnosis and treatment for GAS pharyngotonsillitis were made in accordance with the results from the throat cultures, prescription of penicillin V would be slightly lower compared to if assessments were made in accordance with the results from RADT. However, in clinical practice waiting for throat culture results is known to increase prescription of unnecessary antibiotics for pharyngotonsillitis [Citation32]. Therefore, even with the possibility of occasional false positive RADTs after recent treatment for GAS, we conclude that using RADT will result in lower antibiotic prescribing compared to using throat cultures for diagnosis. Since we found that the combination of negative RADT and positive throat culture was rare, using RADT to confirm GAS before antibiotic treatment implies a low risk for missing presence of GAS also in patients with recent penicillin V treatment.
Meaning of the study
There was no significant difference in the decline of positive test results from RADT and throat culture for GAS in patients who had recently completed penicillin V treatment for GAS pharyngotonsillitis. Negative RADT with positive throat culture was rare at follow-up within 21 days after initiation of treatment. Hence, RADT for GAS is useful in identifying the presence of GAS also after recent penicillin V treatment.
This study was not designed to determine if GAS remaining after penicillin V treatment represents treatment failure, new infection, or carriers ill from something other than GAS.
Ethical approval
The study was approved by the Regional Ethical Review board in Lund, 25 June 2015 (reference number 2015/396) and was registered in the EU Clinical Trials Register, number EudraCT 2015-001752-30. The study was conducted according to the ethical principles of the Declaration of Helsinki. All participants were informed of the study, both verbally and in writing, and provided written consents before participation. In the case of children, both the child and the guardian/guardians provided consent before participation.
Acknowledgements
The authors acknowledge the personnel at the participating primary healthcare centres, and we would like to thank the patients participating in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tyrstrup M, van der Velden A, Engstrom S, et al. Antibiotic prescribing in relation to diagnoses and consultation rates in Belgium, The Netherlands and Sweden: use of european quality indicators. Scand J Prim Health Care. 2017;35(1):10–18.
- Mehta N, Schilder A, Fragaszy E, et al. Antibiotic prescribing in patients with self-reported sore throat. J Antimicrob Chemother. 2017;72(3):914–922.
- Petersen I, Hayward AC. Antibacterial prescribing in primary care. J Antimicrob Chemother. 2007;60(Suppl 1):i43–7.
- Pelucchi C, Grigoryan L, Galeone C, et al. Guideline for the management of acute sore throat. Clin Microbiol Infect. 2012;18(Suppl 1):1–28.
- NICE guidelines. https://www.nice.org.uk/guidance/ng84
- Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the infectious diseases society of america. Clin Infect Dis. 2012;55(10):1279–1282.
- Handläggning av faryngotonsilliter i öppenvård. 2022. https://www.lakemedelsverket.se/48ff63/globalassets/dokument/behandling-och-forskrivning/behandlingsrekommendationer/behandlingsrekommendation/behandlingsrekommendation-antibiotika-vid-faryngotonsilliter-i-oppenvard.pdf.
- Hawes L, Buising K, Mazza D. Antimicrobial stewardship in general practice: a scoping review of the component parts. Antibiotics. 2020;9(8):498.
- World Health Organization. Global action plan on antimicrobial resistance. 2015. http://apps.who.int/iris/bitstream/10665/193736/1/9789241509763_eng.pdf
- Del Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2006 Oct 18;(4):Cd000023.
- Carapetis JR, Steer AC, Mulholland EK, et al. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–694.
- Spinks A, Glasziou PP, Del Mar CB. Antibiotics for treatment of sore throat in children and adults. Cochr Database Syst Rev. 2021;12(12):CD000023.
- Gunnarsson R, Orda U, Elliott B, et al. C. What is the optimal strategy for managing primary care patients with an uncomplicated acute sore throat? Comparing the consequences of nine different strategies using a compilation of previous studies. BMJ Open. 2022;12(4):e059069.
- Centor RM, Witherspoon JM, Dalton HP, et al. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1(3):239–246.
- McIsaac WJ, Kellner JD, Aufricht P, et al. Empirical validation of guidelines for the management of pharyngitis in children and adults. Jama. 2004;291(13):1587–1595.
- Gunnarsson MS, Sundvall PD, Gunnarsson R. In primary health care, never prescribe antibiotics to patients suspected of having an uncomplicated sore throat caused by group A beta-haemolytic streptococci without first confirming the presence of this bacterium. Scand J Infect Dis. 2012;44(12):915–921.
- Orda U, Mitra B, Orda S, et al. Point of care testing for group A streptococci in patients presenting with pharyngitis will improve appropriate antibiotic prescription. Emerg Med Australas. 2016;28(2):199–204.
- Cohen JF, Pauchard JY, Hjelm N, et al. Efficacy and safety of rapid tests to guide antibiotic prescriptions for sore throat. Cochr Database Syst Rev. 2020;6(6):CD012431.
- Donato LJ, Myhre NK, Murray MA, et al. Assessment of test performance and potential for environmental contamination associated with a point-of-Care molecular assay for group A Streptococcus in an end user setting. J Clin Microbiol. 2019;57(2):e01629–18.
- Gerber MA, Shulman ST. Rapid diagnosis of pharyngitis caused by group A streptococci. Clin Microbiol Rev. 2004;17(3):571–580.
- Dubois C, Smeesters PR, Refes Y, et al. Diagnostic accuracy of rapid nucleic acid tests for group A streptococcal pharyngitis: systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(12):1736–1745.
- Lindbaek M, Hoiby EA, Lermark G, et al. Which is the best method to trace group A streptococci in sore throat patients: culture or GAS antigen test? Scand J Prim Health Care. 2004;22(4):233–238.
- Cohen JF, Cohen R, Bidet P, et al. Rapid-antigen detection tests for group a streptococcal pharyngitis: revisiting false-positive results using polymerase chain reaction testing. J Pediatr. 2013;162(6):1282–1284, 4.e1.
- Sheeler RD, Houston MS, Radke S, et al. Accuracy of rapid strep testing in patients who have had recent streptococcal pharyngitis. J Am Board Fam Pract. 2002;15(4):261–265.
- Gerber MA, Randolph MF, DeMeo KK. Streptococcal antigen in the pharynx after initiation of antibiotic therapy. Pediatr Infect Dis J. 1987;6(5):489–491.
- Beach PS, Balfour LC, Lucia HL. Group A streptococcal rapid test: antigen detection After 18–24 hours of penicillin therapy. Clinical Pediatrics. 1989;28(1):6–10.
- Skoog Ståhlgren G, Tyrstrup M, Edlund C, et al. Penicillin V four times daily for five days versus three times daily for 10 days in patients with pharyngotonsillitis caused by group A streptococci: randomised controlled, open label, non-inferiority study. BMJ. 2019;367:l5337.
- Kuhn S, Davies HD, Katzko G, et al. Evaluation of the strep A OIA® assay versus culture methods: ability to detect different quantities of group A Streptococcus. Diagn Microbiol Infect Dis. 1999;34(4):275–280.
- Cohen JF, Chalumeau M, Levy C, et al. Spectrum and inoculum size effect of a rapid antigen detection test for group A streptococcus in children with pharyngitis. PLoS One. 2012;7(6):e39085.
- Cohen JF, Chalumeau M, Levy C, et al. Effect of clinical spectrum, inoculum size and physician characteristics on sensitivity of a rapid antigen detection test for group A streptococcal pharyngitis. Eur J Clin Microbiol Infect Dis. 2013;32(6):787–793.
- Rubin LG, Mirkin GS. Apparent false positive detection of group a Streptococcus antigen resulting from pharyngeal infection with a nonhemolytic Streptococcus pyogenes. Pediatr Infect Dis J. 2000;19(7):672–674.
- Meier FA, Howland J, Johnson J, et al. Effects of a rapid antigen test for group A streptococcal pharyngitis on physician prescribing and antibiotic costs. Arch Intern Med. 1990;150(8):1696–1700.