Abstract
Objective
There have been few studies predicting institutionalization or death in home care settings. We examined risk factors for nursing home placement (NHP) and death among home care patients.
Design
A prospective one-year follow-up study.
Settings and subjects
Persons aged ≥65 years living in Eastern Finland and receiving regular home care services (n = 293).
Main outcome measures
Risk factors for NHP or death were investigated using Cox proportional hazards model. Explanatory variables included demographics, health status (Charlson Comorbidity Index, CCI), physical (Timed Up and Go, TUG), and cognitive (Mini-Mental State Examination, MMSE) functioning, Basic and Instrumental Activities of Daily Living (BADL, IADL) and mood (Geriatric Depression Scale, GDS-15).
Results
Of the 293 patients (mean age 82.6 years, 70.6% women), 27 (9.2%) moved to a nursing home and 25 (6.9%) died during the follow-up (mean 350 days). The combined outcome of NHP or death was predicted by BADL (HR 0.73, CI 95% 0.62–0.86), IADL (0.75, 0.65–0.87) MMSE (0.92, 0.87–0.96), and TUG (1.02, 1.01–1.03). NHP alone was predicted by BADL (0.62, 0.50–0.78), IADL (0.57, 0.45–0.73), and MMSE (0.88, 0.82–0.94) and mortality by TUG (1.02, 1.01–1.03).
Conclusion
Basic measures of functioning can be used to identify high-risk patients in home care. Decreasing BADL, IADL and MMSE predict NHP and longer TUG-times death within a year.
KEY POINTS
Factors associated with institutionalization or death in community-dwelling older populations are studied comprehensively but far less in known about the risks in home care settings
Lower BADL, IADL and MMSE scores predict NHP, and a longer TUG time predicted death within a one-year timeframe among home care patients.
The basic tests of functioning and mobility can be used to identify high-risk patients in home care.
Identification of high-risk patients may also help in targeting of care and rehabilitation
Introduction
Aging is associated with increased morbidity and vulnerability. In later years of life, physiological changes, chronic diseases, and other health problems tend to accumulate and compromise the health and quality of life of individuals [Citation1]. Despite these problems, most older people prefer to live in their own homes because of greater autonomy, integrity of social networks, and a higher quality of life compared with living in institutional settings [Citation2,Citation3].
Institutionalization has been associated with several negative outcomes such as increased mortality, restricted quality of life as well as questionable quality of care [Citation4,Citation5]. Additionally, nursing home placement (NHP) is expensive both in terms of public and private finances. Home care services for older people aim to compensate for functional losses and prevent further impairments, thus supporting living at home and reducing or delaying NHP and death [Citation6].
Many studies have examined predictors of NHP in community-dwelling older people. Functional impairments, cognitive impairment and prior service use have shown to have predictive value [Citation7–10]. In addition, low self-rated health, a high number of prescribed medicines, low socioeconomic status, diagnosed dementia, cardiovascular diseases, Parkinson´s disease, depression and hip fracture were related to institutionalization [Citation8,Citation11,Citation12]. Low body mass index (BMI), previous falls and frailty have also proven to be risk factors for NHP [Citation13].
Mortality and NHP have common risk factors. Increased disability, cognitive problems, prior service use and low self-rated health as well as cardio- and cerebrovascular diseases, respiratory diseases, diabetes, pressure ulcers and the number or severity of diseases have been associated with mortality among older people [Citation11,Citation14,Citation15]. Poor balance and mobility were also significant predictors of mortality [Citation16–22].
Thus far there have been few studies predicting institutionalization or death among home care patients [Citation23]. In this study we examined risk factors for NHP or death in home care settings. We focused on the role of daily functioning, mobility, mood, and cognitive performance.
Material and methods
Study design and participants
This study was a part of the Finnish Interprofessional Medication Assessment (FIMA) study. The complete study design of the FIMA study has been published previously [Citation24,Citation25].
The FIMA study compared physician-led interprofessional medication assessment and usual care in public home care settings in five areas of Finland. Inclusion criteria for the FIMA study were age ≥65 years and registration with public home care services, and at least one of the following: taking ≥6 medicines daily, having dizziness, orthostatic hypotension or experienced a fall in the previous 12 months. Patients whose medication was not managed by home care were excluded from the FIMA study.
In Finland, the national goal is to enable older people to continue living at home for as long as possible, even up to the end of their lives [Citation26]. Municipalities are responsible for arranging social and health care services, including home care and nursing services. Finnish home care services include support and assistance in activities of daily living, home nursing, physician services, rehabilitation, home hospital services in acute situations and end-of-life care [Citation26]. In 2015 in Finland, 6% of the people aged ≥65 years and 11.8 % of the people aged ≥75 years received regular home care services [Citation27].
The present study focused on home care patients from the city of Savonlinna, Eastern Finland. The complete follow-up data including NHP and deaths were documented in these patients (n = 293, 58.6% of all FIMA study participants). The total number of patients in this particular study was 301 of which eight were excluded due to lack of follow-up information (n = 5), death soon after enrollment (n = 2), or lack of baseline information (n = 1).
Preliminary analyzes showed that the randomization status (medication assessment intervention or usual care) was not associated with NHP or death and, therefore, we combined the intervention and usual care groups in the present analyses. The post-hoc statistical power in the present FIMA subsample exceeded 80% in the measures of functioning and mood (see Supplementary Material with Supplementary Figure 1 for a detailed description).
Data collection
An interprofessional team, consisting of a pharmacist, physician and nurses, conducted the data collection between February and December 2015. Registered nurses checked the eligibility of patients and carried out baseline examinations within two weeks after inclusion to the study. The follow-up data were obtained from the records of the East Savo Health Care District.
Descriptive variables
Home care nurses interviewed the patients on their demographics, current health status and functioning using structured questionnaires. The prescribed and over-the-counter medicines used regularly or as needed were recorded as well. The pharmacist reviewed patients’ medication lists using five databases, including Meds75+ Database, which supports clinical decision making on rational and safe pharmacotherapy among persons aged 75 years and older. Medicines classified as class D in Meds75+ should be avoided in the care of older people, for their risks usually outweigh their benefits.
The home care physician documented patients’ diagnoses from existing medical records. For the purpose of this study, a modified Charlson Comorbidity Index (CCI) was calculated to describe disease burden among home care patients. We calculated the index using the following diseases with corresponding scores: metastatic or terminal cancer (score of 6), non-metastatic cancer and moderate or severe renal insufficiency (score of 2), coronary artery disease, type 1 or 2 diabetes, chronic asthma or chronic obstructive pulmonary disease, rheumatoid arthritis, peripheral vascular disease, cerebrovascular disease, dementia of any type or history of gastrointestinal bleeding (score of 1). To assess the patients’ Self-Rated Health (SRH), we used a five-point scale: excellent, very good, good, fair, or poor.
Explanatory variables
The Katz Index was used to assess Basic Activities of Daily Living (BADL) and the Lawton and Brody scale Instrumental Activities of Daily Living (IADL). The BADL index involves basic activities such as body care, dressing, toileting, transferring and feeding. The IADL scale covers complex instrumental activities such as using a telephone, shopping, preparing meals, cleaning, washing clothes, using public transport, and managing medication and finances. The maximum score in BADL is six and in IADL eight, with higher scores indicating better performance.
Mobility was assessed with the Timed Up and Go test (TUG). In the test, patients were timed while rising from a seated position in a chair with an arm rest, walking three meters, turning around, walking back, and sitting. The time taken to complete the TUG test correlates with the level of functional mobility with increasing time indicating worse performance.
To screen cognitive functions, we used The Mini Mental State Examination (MMSE) on a scale from 0 to 30, with higher scores indicating better performance. Depressive symptoms were assessed with the Geriatric Depression Scale (GDS-15), with scores from 0 to 15 and a higher number indicating more depressive symptoms.
Outcomes and follow-up
The follow-up data were collected from the electronic medical records of the East Savo Health Care District. The maximal length of follow-up was one year after the baseline examinations. The study endpoints were dates of NHP or death or the end of the follow-up (whichever came first). We analyzed NHP or death as separate outcomes and as a combined outcome due to the rather low numbers of NHPs and deaths.
Statistical analyses
The Cox proportional hazards model served as a method to investigate associations between the outcome variable, permanent NHP or death, and functional measures. Age, sex, and CCI served as covariates in the Cox models. The randomization status (intervention or usual care) was not associated with NHP or death and, therefore, we combined the intervention and usual care groups in the analyses. We evaluated the proportionality hazards assumption based on the Schoenfeld residuals. For statistical analyses we replaced the missing data of the baseline measurements with the median of the study cohort.
To better demonstrate the association between functioning and NHP or death, we fit a polynomial curve separately for each functional measure and outcome relationship. Moreover, we used a t-test and Mann-Whitney U-test to detect differences in baseline characteristics between patients who were institutionalized or died and those who continued as home care clients during the entire follow-up. IBM SPSS Statistics 27 and GraphPad Prism 5 provided platforms for the statistical analyses.
Results
Participant characteristics
The mean age of patients was 82.6 (range 65–95) years and the majority (70.6%) were women (). At baseline, 73 (24.9%) patients had excellent or very good SRH and 33 (11.3%) had poor SRH.
Table 1. Baseline characteristics of study participants by follow-up status (stays at home or nursing home placement/death).
With regard to morbidity in home care patients, the mean CCI was 2.4 (range 0–11) and the most common diseases were coronary artery disease (41.3%), diabetes (35,8%) and heart failure (34.1%). A fourth of the patients were diagnosed with dementia. All patients had difficulties in daily activities: the average BADL and IADL scores were 4.9 (range 0–6) and 4.1 (range 0–8), respectively. The mean MMSE score was 22.4, ranging from 3 to 30.
The mean number of all medicines taken regularly or as needed was high, 15.2 (range 4–35) and a vast majority of patients (87.4%) used 10 or more medicines. Inappropriate medicines (Meds75+, class D) were used by 73 (24.9%) patients.
Patients who were placed in a nursing home or died during the follow-up were older, had a higher prevalence of diagnosed dementia, scored low in BADL, IADL and MMSE and had a higher score in GDS-15 compared to those who stayed at home. In addition, they performed worse in the TUG test than those who stayed at home.
Risk factors for NHP and death
During the follow-up [mean 350 (SD 8.7) days], 27 (9.2%) patients were moved permanently to nursing homes, 25 (6.9%) died and 241 (82.2%) continued to reside at home. The Cox regression adjusted for age and sex showed that BADL, IADL, MMSE, and TUG explained the risk of combined outcome NHP or death (). The higher the BADL, IADL, and MMSE scores were and the shorter the TUG time was, the lower the risk of NHP or death was. These associations remained significant, when CCI was added to the covariates. The patient who ended up to nursing home or died had more diagnosed dementia but in our analyses no other disease was a risk factor of NHP or death in this study.
Table 2. Cox proportional hazards models for nursing home placement (NHP) or death.
As separate outcomes, the risk of NHP was explained by BADL, IADL, and MMSE, whereas the risk of death was explained by TUG alone (). Regarding the risk of death, however, the effect of TUG was not statistically significant after including CCI in the model (). Depressive symptoms measured with GDS-15 were associated neither with NHP or death nor their combination.
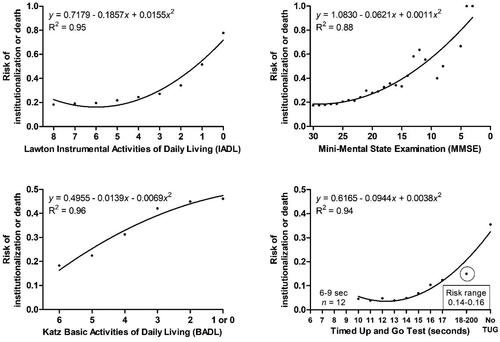
illustrates the relationships between patients’ functional and cognitive test performances at baseline and NHP or death during the follow-up. In general, decreasing levels of daily and cognitive functioning and mobility were associated with an increased risk of NHP or death. In the IADL, the risk increased clearly for scores ≤4. For MMSE, the risk was low for scores 30–25 but increased thereafter. TUG times 14 s or longer represented an increased risk for NHP or death among home care patients. In the BADL, the risk increased almost linearly with a declining score, a 1-point decrease in BADL indicating approximately a 5% increase in the risk.
Discussion
Key findings
We assessed risk factors for NHP or death among home care patients focusing on the role of daily functioning, mobility, mood, and cognitive performance. We measured functioning by indicators that are commonly used in home care practice. All home care patients had varying degrees of disability and needed help in daily activities. Despite the vulnerability and functional limitations, the basic measures of functioning showed to have predictive value in differentiating the risk of NHP and death in home care settings. Measures of daily functioning and cognitive performance proved to be strong predictors of institutionalization. The risk of NHP increased almost linearly with declining BADLs.
Comparison with previous studies
In a meta-analysis with 178,056 older adults (age ≥65 years), people with 3 or more IADL dependencies were approximately three times more likely to enter a nursing home over 2- to 6-year intervals [Citation7]. In a previous Finnish study, IADL scores <5 predicted institutionalization among hip fracture patients aged ≥65 years [Citation9]. In our study, the risk of NHP was quite steady with IADL scores of 8 to 3 and started to increase after the patients had lost their independence in almost all IADLs. Our patients received regular home care and were able to live at home despite having low IADL functioning.
The MMSE is an instrument commonly used for cognitive screening. In this study, the risk of NHP or death started to gradually increase with MMSE scores indicative of mild cognitive impairment. This was concordant with previous findings. For example, in a one-year follow-up after a hip fracture, the MMSE cut-off value of 20 indicated a strong connection with institutionalization [Citation9]. In another study, patients with mild Alzheimer’s disease (AD, MMSE 20–23) already had a higher risk of NHP than patients with early AD (MMSE 27–30) [Citation28]. As well known, progressive cognitive decline also affects functioning in daily activities [Citation8].
We assessed mobility with the TUG test, and it appeared to be the only functional measure which predicted death among home care patients. In earlier studies low walking speed and poor balance were both significant predictors of mortality in community-dwelling older people [Citation16–18,Citation22], and longer TUG times were associated with mortality in both home-dwelling males and females [Citation17,Citation21]. In diagnose-specific populations, for example, among patients with chronic kidney disease, each one-second increase in TUG time was associated with an 8% greater risk of death over the following three years [Citation20]. In a prospective cohort study of patients aged ≥65 years undergoing colorectal or cardiac operation, a preoperative TUG time >15 s was associated with a significantly higher one-year mortality [Citation19].
In our study, the risk of death began to increase after the patient’s TUG time was >13 s but the effect did not remain significant in comorbidity-adjusted analyses. The CCI was originally developed to measure the burden of comorbidity to predict mortality [Citation29]. This may indicate over-adjustment in our mortality analysis, and on the other hand, also reflect the impact of comorbidity on mobility.
Depressive symptoms did not predict NHP or death in our study. In earlier studies, depressive symptoms increased the risk of NHP in primary care patients with mild cognitive impairment or dementia (age 75–81 years) and a concordant association was found in a Finnish register-based study of home-dwelling older people (age ≥65 years) [Citation11,Citation12]. Two reviews concluded that diagnosed depression was a risk factors for NHP or death among home-dwelling older people but there were also studies with unclear or opposite findings [Citation8,Citation11].
Our findings were consistent with earlier prospective studies among home-dwelling older populations showing that institutionalization was associated with cognitive and/or functional impairments [Citation7–10] and functional disability was connected to mortality [Citation14]. Concordantly, a meta-analysis of studies that predicted institutionalization in the U.S. showed that BADLs predicted NHP better than IADLs [Citation7] Functional impairment is first evident in IADLs and later in BADLs [Citation30]. Between cognitive and functional performances exist a strong one-sided association: progressive cognitive decline affects daily functioning, first IADLs and then BADLs [Citation8]. Difficulties in mobility are often the first sign of functional decline and may indicate that a person could benefit from preventive actions and rehabilitation [Citation8,Citation11,Citation17].
Strengths and limitations
Our study has several strengths. The participants were patients of public home care without exclusions regarding morbidity or functionality. The functional measurements used in this study are cheap and commonly used in home care settings and therefore we assume that our findings are generalizable to the home care context. The exact dates of NHP and death were obtained from local registers, and all patients lived in the same area so the criteria and opportunities for NHP were similar for all.
There were also some limitations. The study cohort was quite small, and the follow-up time was quite short (maximum one year) so the number of NHPs and deaths remained low. On the other hand, the short follow-up time strengthened the association between the functional measurements and outcomes. The data were collected from one area in Finland but due to the national home care quality recommendations, the findings are generalizable to the whole country and probably more broadly, at least, to home care settings in other Scandinavian countries, where the services are comparable to ours.
Conclusions and implications
Policymakers and clinicians are challenged with developing and providing services with limited resourced for a growing number of older people. This often means a necessity to focus on high impact problems. Commonly used and validated tests of functioning and mobility proved to be indicators of emerging adverse outcomes, institutionalization and death, among home care patients. Lower scores of BADL, IADL and MMSE predicted NHP and longer TUG times predicted death within a one-year timeframe. The predictive accuracy of these tests in differentiating the risks of NHP and death was good even in this vulnerable patient group.
Developing prediction models is one way to help policymakers and clinicians to do the right things at the right time. Regular screening of home care patients with TUG and at least with one other test of functioning can be used to identify patients at high risk of NHP or death. Detection of high-risk patients may also help in the targeting care and rehabilitation. More research is needed to determine which specific home care services could help to delay institutionalization and death.
Ethical approval
The Research Ethics Committee of Northern Savo Hospital District and Kuopio University Hospital approved the FIMA study protocol on February 3, 2015. All participants or their closest proxy, if the patient had cognitive impairments, gave written informed consent to the study. The FIMA study was registered with ClinicalTrials.gov on March 20, 2015 (identifier: NCT02398812).
Supplemental Material
Download MS Word (242.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Navaratnarajah A, Jackson SH. The physiology of ageing. Medicine. 2017;45(1):6–10. doi: 10.1016/j.mpmed.2016.10.008.
- Harrefors C, Sävenstedt S, Axelsson K. Elderly people’s perceptions of how they want to be cared for: an interview study with healthy elderly couples in Northern Sweden. Scand J Caring Sci. 2009;23(2):353–360. doi: 10.1111/j.1471-6712.2008.00629.x.
- Cheek J, Ballantyne A, Byers L, et al. From retirement village to residential aged care: what older people and their families say. Health Soc Care Commun. 2007;15(1):8–17.
- Olsen C, Pedersen I, Bergland A, et al. Differences in quality of life in home-dwelling persons and nursing home residents with dementia–a cross-sectional study. BMC Geriatr. 2016;16(1):137. doi: 10.1186/s12877-016-0312-4.
- Wolinsky FD, Callahan CM, Fitzgerald JF, et al. The risk of nursing home placement and subsequent death among older adults. J Gerontol. 1992;47(4):S173–S82. doi: 10.1093/geronj/47.4.s173.
- Mayo-Wilson E, Grant S, Burton J, et al. Preventive home visits for mortality, morbidity, and institutionalization in older adults: a systematic review and meta-analysis. PLOS One. 2014;9(3):e89257. doi: 10.1371/journal.pone.0089257.
- Gaugler JE, Duval S, Anderson KA, et al. Predicting nursing home admission in the US: a meta-analysis. BMC Geriatr. 2007;7(1):13. doi: 10.1186/1471-2318-7-13.
- Luppa M, Luck T, Weyerer S, et al. Prediction of institutionalization in the elderly. A systematic review. Age Ageing. 2010;39(1):31–38. doi: 10.1093/ageing/afp202.
- Hongisto MT, Nuotio M, Luukkaala T, et al. Does cognitive/physical screening in an outpatient setting predict institutionalization after hip fracture? BMC Musculoskelet Disord. 2016;17(1):444. doi: 10.1186/s12891-016-1272-8.
- Toot S, Swinson T, Devine M, et al. Causes of nursing home placement for older people with dementia: a systematic review and meta-analysis. Int Psychogeriatr. 2017;29(2):195–208. doi: 10.1017/S1041610216001654.
- Miller EA, Weissert WG. Predicting elderly people’s risk for nursing home placement, hospitalization, functional impairment, and mortality: a synthesis. Med Care Res Rev. 2000;57(3):259–297. doi: 10.1177/107755870005700301.
- Nihtilä EK, Martikainen PT, Koskinen SV, et al. Chronic conditions and the risk of long-term institutionalization among older people. Eur J Public Health. 2008;18(1):77–84. doi: 10.1093/eurpub/ckm025.
- Buys DR, Roth DL, Ritchie CS, et al. Nutritional risk and body mass index predict hospitalization, nursing home admissions, and mortality in community-dwelling older adults: results from the UAB study of aging with 8.5 years of follow-up. J Gerontol Ser Biomed Sci Med Sci. 2014;69(9):1146–1153.
- Millán-Calenti JC, Tubío J, Pita-Fernández S, et al. Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Arch Gerontol Geriatr. 2010;50(3):306–310. doi: 10.1016/j.archger.2009.04.017.
- Landi F, Liperoti R, Russo A, et al. Disability, more than multimorbidity, was predictive of mortality among older persons aged 80 years and older. J Clin Epidemiol. 2010;63(7):752–759. doi: 10.1016/j.jclinepi.2009.09.007.
- Blain H, Carriere I, Sourial N, et al. Balance and walking speed predict subsequent 8-year mortality independently of current and intermediate events in well-functioning women aged 75 years and older. J Nutr Health Aging. 2010;14(7):595–600. doi: 10.1007/s12603-010-0111-0.
- Bergland A, Jørgensen L, Emaus N, et al. Mobility as a predictor of all-cause mortality in older men and women: 11.8 year follow-up in the Tromsø study. BMC Health Serv Res. 2017;17(1):22. doi: 10.1186/s12913-016-1950-0.
- Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341(1):c4467–c4467. doi: 10.1136/bmj.c4467.
- Robinson TN, Wu DS, Sauaia A, et al. Slower walking speed forecasts increased postoperative morbidity and one-year mortality across surgical specialties. Ann Surg. 2013;258(4):582–590. doi: 10.1097/SLA.0b013e3182a4e96c.
- Roshanravan B, Robinson-Cohen C, Patel KV, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24(5):822–830. doi: 10.1681/ASN.2012070702.
- Ascencio EJ, Cieza-Gómez GD, Carrillo-Larco RM, et al. Timed up and go test predicts mortality in older adults in Peru: a population-based cohort study. BMC Geriatr. 2022;22(1):61. doi: 10.1186/s12877-022-02749-6.
- Idland G, Engedal K, Bergland A. Physical performance and 13.5-year mortality in elderly women. Scand J Public Health. 2013;41(1):102–108. doi: 10.1177/1403494812466460.
- Badia JG, Santos AB, Segura JCC, et al. Predictors of mortality among elderly dependent home care patients. BMC Health Serv Res. 2013;13(1):316. doi: 10.1186/1472-6963-13-316.
- Auvinen K, Räisänen J, Merikoski M, et al. The finnish interprofessional medication assessment (FIMA): baseline findings from home care setting. Aging Clin Exp Res. 2019;31(10):1471–1479. doi: 10.1007/s40520-018-1085-8.
- Auvinen K. Interprofessional medication assessment in older people - Findings from Finnish home care [Doctoral dissertion]. Publication of the University of Eastern Finland. https://erepo.uef.fi/bitstream/handle/123456789/28451/urn_isbn_978-952-61-4658-4.pdf?sequence=1.
- Finnish Institute for Health and Welfare. Home care – THL [Internet]. Finnish Institute for Health and Welfare. 2022. https://thl.fi/en/web/ageing/older-people-services-undergoing-a-change/home-care.
- Väyrynen R, Kuronen R. Kotihoidon asiakkaat marraskuussa. 2015. https://www.julkari.fi/bitstream/handle/10024/130786/Tk08_16.pdf?sequence=1.
- Nielsen ABS, Siersma V, Waldemar G, et al. Poor self-rated health did not increase risk of permanent nursing placement or mortality in people with mild Alzheimer’s disease. BMC Geriatr. 2021;21(1):386. doi: 10.1186/s12877-016-0262-x.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8.
- Fieo RA, Austin EJ, Starr JM, et al. Calibrating ADL-IADL scales to improve measurement accuracy and to extend the disability construct into the preclinical range: a systematic review. BMC Geriatr. 2011;11(1):42. doi: 10.1186/1471-2318-11-42.