ABSTRACT
In UK forestry, the synthetic pyrethroid insecticides alpha-cypermethrin and cypermethrin have been used for many years to provide protection for young trees from damage by the large pine weevil, Hylobius abietis L. However, concerns over the toxicity of these insecticides has led to a search for alternatives. In our work, applications of 0.037 g.a.i.stem–1 acetamiprid provided high levels of protection from Hylobius browsing, comparable to alpha-cypermethrin or cypermethrin, and without causing phytotoxicity. However, re-application is likely to be required in the second growing season after planting. Our research identified that acetamiprid treated trees can be safely cold stored as part of normal nursery practice. Acetamiprid is less toxic than synthetic pyrethroids. We also found that pre-treating trees with 0.016 g.a.i.stem–1 chlorantraniliprole, a relatively low toxicity insecticide, can be equally effective in protecting trees, and that dye markers can be safely used to help target spays. Our testing of physical barriers such as MultiPro®, and to a lesser extent Kvaae® wax, suggests they may have a role as a partial substitute for the use of insecticides in some circumstances in the UK and Ireland, but only as part of an integrated approach where on-site populations of Hylobius are predicted to be low.
Introduction
The large pine weevil (Hylobius abietis L., hereafter referred to as “Hylobius”) is a major pest of coniferous trees used to restock recently clear-felled forest sites in northern Europe, including the UK and Ireland (Långström and Day Citation2007; Willoughby et al. Citation2017). Even-aged high forest silvicultural systems encourage large populations of Hylobius to develop as fresh woody material left on-site after cutting, especially the stumps and root systems of harvested trees, attracts the insect to breed (Eidmann Citation1985). Adults lay eggs in cut stumps, roots and other debris, with larvae hatching soon afterwards. Depending on resource quality, habitat and climate, larval development in the UK usually takes between one and three years before adults emerge to feed on the bark of any newly planted trees (Moore Citation2004; Moore et al. Citation2004). The periodic emergence of large numbers of adults from stumps, coupled with their persistence on-site results in significant damage to newly planted trees of many species, with browsing occurring repeatedly during the first five years of establishment. Without effective control measures, death of transplants due to Hylobius browsing often averages around 50% in the first two years, but mortality rates can reach 100% (Heritage et al. Citation1989; Leather et al. Citation1999). Uneven crop establishment can result from replacing dead plants and successful restocking can be seriously compromised (Willoughby et al. Citation2004). Unsustainable loss of revenue can result (Moore et al., Citationin prep.).
Traditionally, trees in European forests have been protected from Hylobius damage by using insecticides (Långström and Day Citation2007). However, concerns over the impact of pesticides, if misused, on human health, environmental condition and ecological functioning, has led to the exploration and adoption of a range of other approaches. In the UK, an integrated approach to the management of this pest is recommended (Willoughby et al. Citation2004, Citation2017). This includes predicting the likely impacts of Hylobius attack using the Hylobius Management Support System, based on a model of the Hylobius life cycle (Moore Citation2018). Forest managers can then consider the full range of chemical and non-chemical approaches available to prevent insect damage to young trees, if necessary by using different combinations of techniques. Although research into non-chemical approaches is ongoing, currently insecticides still need to be used as part of the integrated management of Hylobius on many sites in the UK and Ireland (Willoughby et al. Citation2017).
Synthetic pyrethroid insecticides including permethrin, alpha-cypermethrin and cypermethrin have been widely used since the 1980s to control insect pests in agricultural and horticultural crops. They act by preventing transmission of impulses along nerves of insects, brought about by cloaking the passage of sodium ions through sodium channels in nerve membranes, resulting in intoxication, rapid knockdown, and insect death (MacBean Citation2012). Pre-treatment of individual young trees with alpha-cypermethrin in an off-site plant nursery after they have been lifted, but immediately before they are dispatched to the forest (typically in the spring), generally provides three to seven months of near complete protection from Hylobius damage when these trees are subsequently planted on restock sites. Because the pre-treated trees are dry, with the insecticide having been absorbed onto the bark before they arrive at the forest, there is almost no risk of exposure to bees, and very little risk of any other environmental contamination. Post-planting sprays of cypermethrin, often called “top-up sprays”, give approximately one to three months additional protection depending on the size of the local Hylobius population. However, cypermethrin is extremely toxic to aquatic life if watercourses are accidentally contaminated through spray drift, run-off, or poor mixing and filling practices (Willoughby et al. Citation2017). Alpha-cypermethrin and cypermethrin are also now classified as “highly hazardous – highly restricted” by the Forest Stewardship Council (FSC), the presumption being that they should not normally be used for post-planting top-up spraying on estates voluntarily certified to FSC standards via the UK Woodland Assurance Standard, where less hazardous alternatives are available (UKWAS Citation2018; FSC Citation2019). In addition, cypermethrin is classified as a priority substance under the European Commission Water Framework Directive (European Commission Citation2013) meaning its usage must be progressively reduced.
The environmental concerns over the use of synthetic pyrethroid insecticides as top-up sprays on forestry restock sites, together with the legal and policy challenges identified above, have led to research across Europe to search for less hazardous alternative methods of tree protection (e.g. Långström and Day Citation2007; Harvey et al. Citation2016). In the UK, a large collaborative research programme took place between 2009 and 2017 involving public forestry administrations across Great Britain, private forestry companies, forest nurseries, and Forest Research scientists. Willoughby et al. (Citation2020) and Moore et al. (Citationin prep.) have described some of the results from this programme, and this paper reports on further experiments in the series which examined various potential chemical and non-chemical replacements for alpha-cypermethrin and cypermethrin use in plantation forestry. This involved five different trials investigating the efficacy and tree tolerance of acetamiprid, chlorantraniliprole, physical barriers and dye markers, on a research enclosure in southern England, and on four typical forest restock sites in north-east England.
Acetamiprid is a broad spectrum, systemic, synthetic neonicotinoid insecticide which affects or kill insects by contact or ingestion (MacBean Citation2012) (see for insecticide product names). It has been widely used since the 1990s to control insect pests in agricultural and horticultural crops, and on home garden plants. In a laboratory study (Olenici et al. Citation2014), acetamiprid was shown to be effective at reducing Hylobius damage to excised twigs but could not prevent damage altogether. Nevertheless, given its broad spectrum of control, the fact that it is readily taken up and translocated by plants, and that it is around 500 times less toxic to aquatic life (Willoughby et al. Citation2017), it was thought to be a good candidate for investigation as a potential replacement for pre-planting and top-up spraying of alpha-cypermethrin and cypermethrin. The experiments reported in Willoughby et al. (Citation2020) and Moore et al. (Citationin prep.) indicated that acetamiprid showed considerable promise for protecting trees, at least over the first year after tree planting. However, further investigation was required into how efficacy declined in the second growing season after treatment, the best method of applying the insecticide, and into what part of young plants should be targeted by sprays.
Table 1. List of products tested.
A further low toxicity synthetic pesticide tested in our work was chlorantraniliprole, which is a selective, translaminar, synthetic diamide insecticide acting by contact and ingestion (MacBean Citation2012). Willoughby et al. (Citation2020) concluded that at some restock sites, chlorantraniliprole offered levels of protection comparable to that of traditional synthetic pyrethroid pesticides. Nevertheless, they suggested that further targeted research should be undertaken to understand better how its use could be optimised.
We were also interested in testing examples of physical barrier systems developed to protect seedlings from weevil browsing, following research which demonstrated their effectiveness in Sweden, usually in combination with other silvicultural measures (e.g. Petersson and Örlander Citation2003; Nordlander et al. Citation2009). Kvaae® wax is a flexible protective wax product developed in Norway, consisting of a mixture of hydrocarbon (petroleum) wax and titanium dioxide, which is applied to the tree stem. MultiPro® is a custom-made short cardboard sleeve manufactured in Sweden, fitted around trees either at the nursery or after planting. Willoughby et al. (Citation2020) reported that these two products did not consistently provide sufficient protection to be viewed as effective substitutes for insecticides on restock sites with high Hylobius population levels, which are commonplace in the UK and Ireland. However, results were sufficiently promising on sites with low to medium population pressure to warrant further testing. We also wished to investigate whether these physical barriers could be used in conjunction with transplants pre-treated with insecticide, to see if such an approach might reduce the need for post-planting top-up spraying of pesticide in the forest, which is potentially more hazardous to the environment than pre-treatment of plants in off-site tree nurseries.
A further aspect of our study was to explore the role and effect of cold storage on the efficacy and phytotoxicity of alpha-cypermethrin, acetamiprid and wax coating of transplants applied in the nursery. Temporary cold storage of seedlings at 0 to +2°C is commonplace in commercial nursery production, as it provides the flexibility to allow plants to be lifted when they are dormant, and then despatched at a later date when conditions become more suitable for planting in the forest (McKay et al. Citation1994; Morgan Citation1999). Sitka spruce (Picea sitchensis (Bong.) Carrière) plants can tolerate cold storage for three months without detriment to their physical condition, as long as they are lifted when they are fully dormant, a state which they typically enter into from mid-November to early December (Morgan Citation1999), and provided they are prevented from desiccating for example by being stored in polythene bags. Previous research has shown that if plants are not fully dormant when they are lifted, then the process of applying insecticides can damage them (Morgan Citation1999), presumably due to mechanical damage of fine roots when the plants are handled (McKay et al. Citation1994). The Electrodyn® conveyor belt spray treatment system was developed by the Forestry Commission in conjunction with ICI plc in the 1990s, and uses insecticides specially formulated with oil and a cyclohexanone organic solvent. Electrostatically charged spray particles are applied to earthed, dormant seedlings placed on a conveyor belt, that then passes through an enclosed spray booth. The charged droplets are attracted to the earthed seedlings, which allows very low volumes of pesticide (0.1 ml tree−1) to be used, whilst still providing good spray coverage of the plants. This reduces the risk of operator contamination, and means the plants dry very rapidly, so they can be immediately bagged for onward dispatch to the forest without the risk of desiccation (Heritage et al. Citation1997). As noted earlier, the use of dry, pre-treated plants for restocking poses a much lower risk of environmental contamination than if the same insecticide was used to make a conventional, post-planting spray in the forest of previously untreated trees. However, research has shown that when permethrin was formulated to allow its use via the Electrodyn® system, the cyclohexanone solvent caused severe damage to plants if they were subsequently cold stored (Morgan Citation1999). For this reason it has been assumed by many nursery producers in the UK that Alpha C 6ED, the Electrodyn® ready insecticide formulation of alpha-cypermethrin that replaced permethrin, would also be phytotoxic to plants if they were cold stored after treatment. Therefore, it is currently standard practice only to treat plants with alpha-cypermethrin in the Electrodyn® immediately before they are dispatched to the forest, whether they are freshly lifted or have been previously cold stored. For our work we were interested in seeing whether plants pre-treated using alternative methods such as coating them with wax, or by using a conventional aqueous spray of the insecticides acetamiprid or alpha-cypermethrin, could subsequently be successfully cold-stored without causing phytotoxicity, or reducing the efficacy of the treatments – if they could this might provide welcome additional operational flexibility to nursery growers.
In summary, the overall objectives of our research programme were to try to identify alternative, effective and safe chemical and non-chemical approaches to the use of synthetic pyrethroid pesticides for protecting young trees from damaging attacks by Hylobius in the UK and Ireland; and to test the hypothesis that cold storage of plants in combination with alternative methods does not adversely affect efficacy, tree health, and survival.
Materials and methods
General design – research enclosure site
Experiments 1 and 2 were situated at Headley Research Enclosure in lowland Britain where, due to the fact that no large scale tree felling had taken place in the enclosure itself or in the surrounding forest for at least a decade, Hylobius pressure was assumed to be negligible or non-existent. This allowed the investigation of treatment phytotoxicity without any confounding effect of any Hylobius browsing damage. In both experiments, assessments of transplant foliage health and survival were made at the end of the first growing season (in October) using a subjective five-point scale (where 1 = healthy; 2 = reasonably healthy, ∼ 25% of foliage discoloured or dead or died back; 3 = moderately healthy, ∼ 50% discoloured etc.; 4 = poor, ∼ 75% discoloured etc.; 5 = dead). Height and root collar diameter were also measured as the start and end of the growing season, to give growth increment.
General design – forest sites
Experiments 3, 4 and 5 were established on sites to be restocked with Sitka spruce. Although all young trees of all species can be damaged by Hylobius browsing, Sitka spruce represents the most important commercial conifer in Great Britain, covering 51% of all coniferous forests (Forestry Commission Citation2019). Sitka spruce is also an important species in Ireland and other north-western Europe countries (Houston Durrant et al. Citation2016). Effectiveness of the different treatments (see for a full list of the products tested) was determined by comparing the amounts of damage to planted trees. Based on their location and timing of felling, the four sites (see ) were selected so that predicted over-wintering adult populations of Hylobius would be relatively low during the first few months of the spring following planting, but numbers would increase to form a substantial population likely to cause significant damage following the emergence of new adults from stumps of the previous crop from August onwards (Moore Citation2004). Thus it was intended that damage would develop gradually over the first three months after planting and then accumulate more rapidly during the following three months of emergence, to provide a representative test of the efficacy of the insecticide treatments under the type of severe feeding pressure likely to be regularly faced on more challenging restocking sites in the UK and Ireland. All the forest sites had previously had a standing crop of at least 70% Sitka spruce and had been harvested 8–18 months before the start of the experiments.
Table 2. Experiment site details.
After clear-felling, the sites were prepared for restocking by excavator mounding cultivation (Paterson and Mason Citation1999), producing a settled mound size of approximately 50 × 50 × 20 cm. This promoted successful establishment by providing an elevated, aerated planting position clear of weed vegetation that might otherwise compete with the seedlings for moisture and nutrients. In addition, there is evidence that adult weevils are discouraged from stopping to feed on plants surrounded by bare mineral soil exposed by cultivation, which in turn can help to reduce browsing pressure on young seedlings, particularly when combined with other protective measures (Örlander and Nilsson Citation1999; Kindvall et al. Citation2000; Björklund et al. Citation2003; Petersson et al. Citation2005). Planting was carried out during December – May depending on site (see ). Two-year-old bare-root Sitka spruce transplants, ranging from 18 to 40 cm in height, were planted. Nursery stock were not treated with any insecticides in the three months prior to the experimental treatment and planting, and none, apart from the experimental treatments, were applied after planting. The transplants were planted at 2 m spacing, with 25 plants per treatment plot. This resulted in a 5 × 5 tree grid and a plot area of roughly 100 m2. Where possible, plots in the same block were separated by a 4 m wide unplanted buffer, whilst those in different replicate blocks were separated by an 8 m wide unplanted buffer. All transplants in a plot were assigned the same plot treatment. A 10 m wide unplanted buffer strip was established around the perimeter of each experimental area to eliminate unwanted effects from off-site operations (e.g. chemical spray-drift, leaching). An untreated control was also included on all sites as an indicator of Hylobius population size and damage pressure, as well as to evaluate the efficacy of the experimental treatments. Unless noted otherwise, experimental treatments were applied by spot gun or knapsack sprayer at Delamere tree nursery, Cheshire, after the trees were lifted, but prior to their despatch for planting in the forest, usually at a spray volume rate of between 10 and 20 ml per plant, to achieve complete and even coverage of the stem down to the root collar.
A standard Hylobius damage scoring system was used (Heritage et al. Citation1989). Using this method, transplants were scored in July and October as follows:-
Alive, undamaged by Hylobius.
Alive, but with signs of sub-lethal Hylobius damage (without cambial girdling).
Dead, killed by Hylobius action (usually with cambial girdling).
Dead, killed by means other than Hylobius (e.g. transplant failure, mammal browsing).
Transplant missing.
Specific design for Experiment 1 – effect of cold storage on the phytotoxicity of pre-treatment with alpha-cypermethrin, acetamiprid, or barrier wax
The aim of this experiment was to investigate the phytotoxicity of pre-treating Sitka spruce plants with alpha-cypermethrin, acetamiprid and the physical protection product Kvaae® wax, and how any phytotoxicity was affected by cold storage. The experiment was carried out at Headley Research Enclosure in Hampshire, south-east England (see for site details). The efficacy of these treatments in preventing Hylobius browsing was tested separately – see Experiment 3.
To provide the plants for both Experiments 1 and 3, two-year-old bare-root Sitka spruce transplants, ranging from 17 to 30 cm in height (mean height 22 cm, mean root collar diameter 2.6 mm), were lifted when dormant from propagation beds at Delamere tree nursery, Cheshire, in December 2014, and then left untreated or pre-treated with either alpha-cypermethrin, acetamiprid, or Kvaae® wax. The two insecticides were applied by knapsack sprayers, and the Kvaae® wax was applied to 50% of the stem and foliage (measured from the root collar) by dipping plant parts using a wax fountain machine (Heco-V-450NW, ZetaEcotech). Untreated and pre-treated trees were then randomly assigned to one of three cold storage / planting groups: – (1) No storage – planted immediately; (2) Cold stored at 0–2°C in opaque, co-extruded polythene bags for 1.5 months – planted in January 2015; or (3) Cold stored as (2) but for three months – planted in March 2015. This created 12 different experimental treatments in total – see and for details. Half of the plants were used in Experiment 1 at Headley, and half of the plants at Experiment 3 at Kielder Forest (see later). Sample plants from each treatment were subject to a Root Electrolyte Leakage (REL) test immediately before dispatch, following the procedure outlined by McKay (Citation1992), to give an indication of both dormancy status, and transplant health, at the point of planting.
Table 3. Experimental treatments at Headley Research Enclosure (Experiment 1), and Unicorn Knowe, Kielder (Experiment 3).
For Experiment 1, three replicated blocks were established at Headley Research Enclosure, each containing 12, randomly located experimental treatment plots. Each 3 m × 3.5 m treatment plot contained 25 trees, at 1.5 m × 0.5 m spacing. There was a 1.5 m buffer between trees of different treatments. The site was cleared of vegetation before planting, fertilised with potassium sulphate to overcome the measured soil potassium deficiency, and kept weed-free throughout the growing season by carefully directed sprays of glyphosate and hand weeding. Plants were irrigated as necessary during periods of dry and hot weather to prevent water stress developing. Transplant growth increment and health were assessed at the end of the first growing season, as described earlier. A full list of products and treatments is given in and .
Specific design for Experiment 2 – tolerance of transplants to pre-treatment with barrier wax
The principal aim of this experiment was to test the phytotoxicity of different coverage levels of barrier wax applied to small and large nursery-grown transplants of seven tree species. This experiment was also carried out at Headley Research Enclosure (see ) to avoid the potential confounding effect of Hylobius browsing damage. Earlier research had shown that whilst wax treatment had some potential for reducing Hylobius browsing on forest sites, depending on the coverage of the plant it could also suppress growth, which could then lead to increased mortality, particularly in smaller trees (Willoughby et al. Citation2020). In addition, anecdotal evidence from nursery growers suggested that different species and plant types could be affected differently. In Experiment 2 we therefore tested the tolerance of Sitka spruce, Norway spruce (Picea abies (L.) H. Karst.), western red cedar (Thuja plicata Donn ex D. Don), Scots pine (Pinus sylvestris L.), Douglas fir (Pseudotsuga menziesii (Mirbel) Franco) and pedunculate oak (Quercus robur L.). As well as conventionally produced bare-root transplants, we also tested Sitka spruce plants that had been produced by vegetative propagation from cuttings, which for the purposes of the experimental design we classed as a seventh “species”. For each species, it was originally intended that two size classes of transplants would be used, categorised as “small” and “large”, but “large” Norway spruce and Scots pine, and “small” Western red cedar and oak, were not available – see for the actual seedling sizes used. Kvaae® wax was applied to cover either 0%, 50%, 75% and 100% of the stem and foliage, as measured from the root collar upwards. The terminal bud was not treated. This gave 40 different treatments in total, which were randomly allocated to three replicate blocks – see and for full details. All other methods were as for Experiment 1.
Table 4. Experimental treatments at Headley Research Enclosure (Experiment 2).
Specific design for Experiment 3 – effect of cold storage on the efficacy of pre-treatment with alpha-cypermethrin, acetamiprid, or barrier wax
The aim of this experiment was to investigate the efficacy of pre-treatment with alpha-cypermethrin, acetamiprid and the physical protection product Kvaae® wax in protecting Sitka spruce plants from Hylobius damage, and how any efficacy was affected by cold storage. The experimental treatments were the same as those tested in Experiment 1, except that the transplants were established at Unicorn Knowe in Kielder Forest, on a clear-fell site which was expected to have a high population of Hylobius. Eight replicate trial blocks were established, each of which contained 12 randomised treatment plots. All trees were planted prior to the predicted commencement of Hylobius browsing. Transplant damage assessments were made at the end of the first and second growing seasons. A full list of products and treatments is given in and . All other methods were as described earlier under “General design – forest sites”.
Specific design for Experiment 4 – efficacy of pre-treatment with acetamiprid, synthetic pyrethroids and physical barriers applied by different methods
The aim of this experiment was to determine the most effective combination of insecticide concentration and spray delivery volume for applying a standard dose of acetamiprid to Sitka spruce transplants, and also to test the importance of the parts of the plant targeted for spray coverage during application (i.e. the whole tree including roots, or stem and foliage only). In addition, the experiment investigated the efficacy of different levels of wax coverage of stems (in a similar way as Experiment 2 had tested the phytotoxicity of increased levels of wax coverage). The MultiPro® physical barrier sleeve was also tested. Both of the physical barrier systems were also combined with insecticide treatments, to see if this integrated approach might result in a reduced need for top-up sprays of insecticide in the forest. Different sizes of Sitka spruce plants were used – “small” (22–25 cm) and “large” (32–37 cm), but both had root collar diameters in the range of 2–7 mm – see . Vegetatively propagated Sitka spruce were also used with MultiPro® guards, as it was thought that the use of this plant type, which typically has far fewer side branches than conventionally produced transplants, might overcome some of the previously reported issues with fitting these types of guards over young trees (Willoughby et al. Citation2020). All treatments were applied in the nursery after trees had been lifted, but prior to dispatch to the forest, except for:- (i) cypermethrin which was applied immediately post-planting in the forest (treatments 5 and 6); (ii) the combination of wax and insecticide, where wax was applied first in the nursery, then acetamiprid was sprayed over untreated parts of the foliage after planting in the forest (treatments 18 and 22); (iii) the combination of MultiPro® guards and insecticides, where acetamiprid was applied to the trees immediately after planting with the sleeves temporarily removed (treatment 25); and (iv) the in-bed treatments of acetamiprid, where the insecticide was applied over the transplant beds of Sitka spruce before lifting (treatments 13 and 14). All insecticide treatments made to individual stems (i.e. not treatments 13 and 14), were at a volume rate (insecticide plus diluent) of 20 ml stem−1, except for treatments 7 and 10 where 5 ml stem−1 was used, and treatments 8 and 11 where 10 ml stem−1 was used: in these exceptions, the concentration of the mix was varied so that the amount of acetamiprid applied to each stem remained the same (0.037 g a.i. stem−1 acetamiprid).
Table 5. Experimental treatments at Binky Burn and Wainhope Linn, Kielder Forest (Experiment 4).
The experiment was carried out at two sites, Binky Burn and Wainhope Linn, which were both located in Kielder Forest, north-east England. At each site four replicate trial blocks were established, each containing 25 randomised plots. Assessments were carried out in the middle of the growing season over two years. A full list of products and treatments is given in and . All other methods were as described earlier under “general design – forest sites”.
Specific design for Experiment 5 – efficacy of pre-treatment with acetamiprid or chlorantraniliprole
This experiment was designed to investigate the effect of dose rate on the efficacy of chlorantraniliprole, applied as a pre-treatment in the nursery, in protecting young Sitka spruce trees that were subsequently planted out in the forest. The impact on efficacy of adding a dye marker, which is often mixed with diluent when top-up applications are made in forests to make the resulting spray mist more visible to operators, and hence help them to minimise drift, contamination, and non-target runoff (Brown et al. Citation2003), was also tested. In addition, the effectiveness of acetamiprid applied via a new pre-treatment machine developed by the Forestry Commission was examined, to see if the resulting efficacy in protecting planting stock from Hylobius browsing damage in the forest matched that expected from standard research treatments applied by knapsack sprayers. Unfortunately, in this experiment, an error was made in calibration of the prototype machine such that only a quarter of the intended acetamiprid dose was applied, so direct comparisons between machine and knapsack sprayer treated plants were not possible. All planting stock used was in the range of 18–25 cm tall, with root collar diameters of 2–3 mm.
The experiment was established at Stourcleugh in Spadeadam Forest, north-east England () and it consisted of 11 treatments arranged in eight, fully randomised blocks. Assessments were carried out over two growing seasons. A full list of products and treatments is given in and . All other methods were as described earlier under “General design – forest sites”.
Table 6. Experimental treatments at Stourcleugh, Spadeadam Forest (Experiment 5).
Data analysis
Statistical analysis for all experiments was conducted using R (version 3.5.2) (R Core Team Citation2018).
For Experiments 1 and 2, tree health scores were converted to binary responses (tree health scores 1 or 2 = 1; tree health scores 3 or 4 or 5 = 0) and modelled using generalised linear mixed effects models with probit link function and binomial errors. For Experiment 2, tree size and species were not balanced (i.e. due to issues with plant supply, not all species had both small and large size classes), therefore these were combined into a single tree species × tree size factor. The main effect of block and interaction between all other factors were included within initial models, with plot nested within block as a random factor. Significant factors and variables were determined using Akaike step functions and significance based on ANOVA-type analysis (Fox and Weisberg Citation2011), using Likelihood Ratio chi-square statistics. Post hoc marginal means were calculated by treatment using the emmeans package (Lenth et al. Citation2019), with contrasts between treatments corrected for multiple comparisons using Tukey’s HSD; for the purposes of presentation, time was set to the latest date at which full treatment assessment was conducted for these marginal mean comparisons.
For height and RCD growth increment analysis in Experiments 1 and 2, linear mixed effects models were fitted to the data (with appropriate assumption checking), with plot nested within block as a random factor. Significant factors and variables were determined using ANOVA F tests with Kenward-Roger density degrees of freedom (Kuznetsova et al. Citation2017). Post hoc analysis followed similar procedures as for tree health.
Data analyses for Experiments 3, 4 and 5 were based on the number of plot transplants assigned to each damage category as described earlier. This procedure was carried out for each experiment, and for each site where an experiment was established on more than one site.
Percentage transplant survival, which is used in this paper as a measure of efficacy, was calculated as the number of trees still alive (categories A + B) divided by the number of trees available to Hylobius (categories A + B + C). For descriptive outputs, survival was multiplied by 100 to obtain percentages. For Experiment 3 only, the analysis was also repeated to include those trees initially categorised as being dead due unknown causes other than Hylobius (D), and missing trees (X), as part of the total number of trees available to Hylobius (data not shown).
Data were analysed using a generalised linear model with logit link and quasibinomial errors (to account for overdispersion). The initial model included block nested within site along with three-way interactions between site, treatment and time (year standardised to a linear unit of 1). Significant factors and variables were determined using Akaike step functions and significance based on ANOVA-type analysis (Fox and Weisberg Citation2011), using Likelihood Ratio chi-square statistics.
Post hoc analysis followed similar procedures as for tree health. However, for Experiments 3, 4 and 5, plot survival data were in addition tested in a pairwise comparison against the untreated control, and in Experiment 4 also against a single active control, alpha-cypermethrin (Alpha C 6ED®, applied via the Electrodyn® system), as this is a commonly used, industry standard, nursery applied insecticide treatment.
Results
Experiment 1
There was no evidence of any Hylobius browsing damage indicating, as expected, that Hylobius was not present in any significant numbers at Headley Research Enclosure, and no obvious signs of insecticide phytotoxicity (such as twisted leaves, stunting, or chlorosis). Root Electrolyte Leakage values calculated at the time of each planting date () were all less than 25%, which is the maximum value that would normally be expected to ensure healthy trees likely to survive and thrive after planting (Morgan Citation1999), except for the alpha-cypermethrin treated trees that were stored for 1.5 months (treatment 5), which had a marginally higher mean REL value of 29%. By the end of the first growing season after planting, there were no differences in tree health amongst the three unprotected (control) treatments (treatments 1–3), where only length of cold storage / planting date was varied (). For those treated with alpha-cypermethrin, there was a significant (p < 0.05) reduction in health in the trees planted in December 2014 (0 months cold storage, treatment 4) compared to those stored for three months and planted in March (treatment 6), or to the 0 months untreated control. Similarly, the health of trees treated with acetamiprid and then immediately planted without cold storage (treatment 7), was significantly worse (p < 0.05) than the equivalent untreated control plants. Application of Kvaae® wax (treatments 10–12) made no significant difference to health compared to the untreated controls.
Figure 1. Transplant status after one growing season at Headley Research Enclosure, Experiment 1. Notes: (A) Distribution of tree health scores (THS) (1 = healthy; 5= dead) and (B) height growth increment (cm) for Sitka spruce transplants planted in December 2014 (0 months storage), January 2015 (1.5 months storage) or March 2015 (3 months storage) and treated with alpha-cypermethrin, acetamiprid, or a Kvaae® wax barrier coating. For health score data, lettering indicates significant differences in proportion of trees with THS of 1/ 2 versus 3/4/5. For height growth data, lettering indicates significant differences in height growth across all treatments (corrected for multiple comparisons). Error bars = 95% confidence intervals (uncorrected).
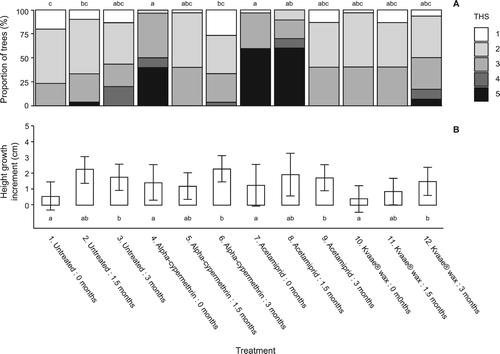
No differences in height increment were found between the untreated control, alpha-cypermethrin, acetamiprid, or Kvaae® wax treated plots, but growth was significantly (p < 0.05) greater in all treatments where trees were planted in March, after three months cold storage compared to the equivalent treatment planted without storage.
Experiment 2
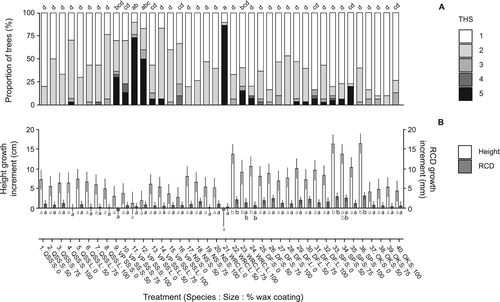
Again, as expected, there was no evidence of any Hylobius browsing. Increasing Kvaae® wax coverage appeared to increase mortality and / or worsen transplant health in a few cases such as for small and large, conventionally produced Sitka spruce transplants (treatments 1–8), and small vegetatively propagated Sitka spruce transplants (treatments 9–12). However, apart from a worsening in health between 50 and 75% wax coverage in the vegetatively propagated stock (treatments 10 and 11), these results were not statistically significant (). There was also no significant difference in transplant health between different sizes of conventional Sitka spruce transplants. However, larger vegetatively propagated Sitka spruce (treatments 15 and 16) were significantly (p < 0.05) healthier than smaller plants (treatments 11 and 12) at higher wax coverage levels (75 and 100%). Overall though, wax application was found to be generally non-toxic to the species tested, albeit with a suggestion that tree health might potentially in some cases start to worsen with higher rates of coverage. Growth data revealed no significant or meaningful results or trends.
Figure 2. Transplant status after one growing season at Headley Research Enclosure, Experiment 2. Notes: (A) Distribution of tree health scores (THS) (1 = healthy; 5= dead) and (B) height and root collar diameter (RCD) growth (cm) for transplants treated with Kvaae® wax coating covering 0%, 50%, 75% and 100% of the length of the stem. For health score data, lettering indicates significant differences in proportion of trees with THS of 1/2 versus 3/4/5. For growth data, lettering indicates significant differences in height and RCD growth within each species/size combination (corrected for multiple comparisons). Error bars = 95% confidence intervals (uncorrected). Species codes are: QSS, Sitka spruce (seed orchard); VPSS, Sitka spruce (vegetatively propagated); NS, Norway spruce; WRC, western red cedar; DF, Douglas fir; SP, Scots pine; OK, pedunculate oak.
Experiment 3
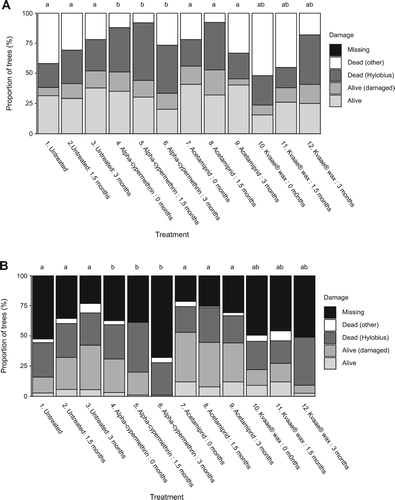
At the end of the first growing season, tree survival in the untreated control plots at Unicorn Knowe was low (c. 42%), suggesting the site had a high population of Hylobius (treatments 1–3, a). Date of planting / length of cold storage had no significant impact on transplant survival in any of the treatments. Unexpectedly, treatment with alpha-cypermethrin (treatments 4–6) resulted in significantly (p < 0.05) poorer survival compared with the untreated controls. Acetamiprid (treatments 7–9) gave significantly (p < 0.05) better protection compared to alpha-cypermethrin treated plots, though survival rates were only around 50% which was not significantly different to the untreated controls. Transplants treated with Kvaae® wax (treatments 10–12) fared no better than those in the untreated controls. In 2016, after two growing seasons (b), the statistical differences between alpha-cypermethrin and acetamiprid treatments were sustained but much less evident. However, there were clearly far fewer missing trees in the acetamiprid plots, and if this is assumed to be due to Hylobius browsing rather than as a result of unproven causes, this would imply that around 50% of the trees initially planted were still being protected by acetamiprid after two growing seasons, and that this insecticide was providing significantly (p < 0.05) better protection than alpha-cypermethrin or Kvaae® wax.
Figure 3. (A) Transplant status after one growing season at Unicorn Knowe, Kielder Forest in 2015, Experiment 3. Notes: Sitka spruce transplants planted in December 2014 (0 months storage), January 2015 (1.5 months storage) or March 2015 (3 months storage) and treated with alpha-cypermethrin, acetamiprid, or a Kvaae® wax barrier coating. Lettering indicates a statistical difference in the proportion of treatment survival (alive/alive (damaged) versus dead (Hylobius), corrected for multiple comparisons (note: tested at the treatment level, as there was no significant treatment : storage interaction). Other mortality and missing transplants were excluded from the statistical analysis. (B) Transplant status after two growing season at Unicorn Knowe, Kielder Forest in 2016, Experiment 3. Notes: Transplants were planted and treated as for a. Lettering indicates a statistical difference in the proportion of treatment survival (alive/alive(damaged) versus dead (Hylobius), corrected for multiple comparisons (note: tested at the treatment level, as no significant treatment : storage interaction). Other mortality and missing transplants were excluded from the statistical analysis.
Experiment 4
At Binky Burn, the mean survival of small and large transplants in untreated control plots (treatments 1 and 2) in the middle of the first growing season in 2015 was only around 20% and 5%, respectively, implying Hylobius populations were very high (a). At Wainhope Linn, Hylobius feeding pressure was less severe and mean survival amongst the small and large transplants in the untreated control plots was higher (c. 47% and 65% respectively).
Figure 4. (A) Transplant status at Binky Burn and Wainhope Linn, Kielder Forest in mid-2014, Experiment 4. Notes: Transplant status including damage caused by Hylobius, across 25 treatments. Lettering indicates a statistical difference in the proportion of treatment survival (alive/alive(damaged) versus dead (Hylobius)); * = significant difference to active control (p < 0.05; grouped mean of treatment 3, Alpha-cypermethrin small size class, and treatment 4, alpha-cypermethrin large size class); † = significant difference to untreated control (p < 0.05; grouped mean of treatments 1 and 2). Other mortality and missing transplants were excluded from the statistical analysis. Treatment effects were consistent across sites, with main effect of site only (% dead due to Hylobius greater overall at Binky Burn). (B) Transplant status at Binky Burn and Wainhope Linn in mid-2015, Experiment 4. Notes: Transplant status including damage caused by Hylobius, across 13 of 25 treatments (for reasons of economy it was not possible to reassess all treatments, and so the decision was taken to focus only on those which included acetamiprid). Lettering indicates a statistical difference in the proportion of treatment survival (alive/alive (damaged) versus dead (Hylobius)); † = significant difference to untreated control (p < 0.05; grouped mean of treatments 1 and 2). Other mortality and missing transplants were excluded from the statistical analysis. Treatment effects were consistent across sites, with main effect of site only (% dead due to Hylobius greater overall at Binky Burn).
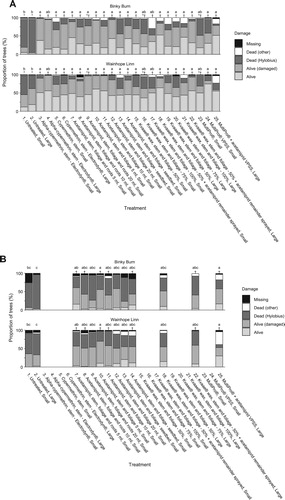
In the first growing season, the patterns of significant differences in survival between treatments were identical in each of the two experimental sites. Compared to the untreated controls, nearly all treatments significantly (p < 0.05) reduced Hylobius damage, and there were no significant differences in survival between transplants of different sizes across the various treatments which examined this.
The industry standard active control alpha-cypermethrin (treatments 3 and 4) provided very effective protection against Hylobius feeding damage, resulting in near total survival (90–100%) across small and large transplants at both sites. Field applications of cypermethrin (treatments 5 and 6) appeared to result in somewhat lower transplant survival than with pre-planting applications of alpha-cypermethrin, but this was not statistically significant.
Acetamiprid (treatments 7–12) was very effective with an average of 90% (range = 74–97%) of plants surviving, and there were no consistent significant differences between treatments that examined application of this insecticide to different parts of the transplant (stem, foliage and roots; versus stem and foliage only). Varying the spray volume rate whilst maintaining the same amount of insecticide active ingredient applied to each stem (0.037 g a.i. ha−1 stem acetamiprid) had no effect on efficacy. Applying acetamiprid to nursery beds before the transplants were lifted (treatments 13 and 14) appeared to result in somewhat lower transplant survival than conventional application to individual stems post lifting (treatments 7–12), but again this was not statistically significant.
Compared to untreated plants, application of Kvaae® wax significantly (p < 0.05) reduced mortality due to Hylobius browsing. Survival (c. 60–85%) was apparently slightly poorer than the insecticide treatments, but the difference was not statistically significant. There was also a suggestion that small transplants might have suffered reduced protection, especially at higher rates of coverage, but again this was not statistically significant. Numbers of missing trees were relatively high, particularly with 100% wax coverage, indicating possible phytotoxic effects. Combining wax with acetamiprid did not improve the protection offered by the insecticide alone.
Although it provided some protection from browsing compared to the untreated controls, only c. 50–70% of smaller, conventionally produced plants in MultiPro® guards survived by the middle of the first growing season across the two sites, and the protection provided was significantly worse than pre-treatment with alpha-cypermethrin. Vegetatively propagated plants fared slightly better (c. 59–85% survival). Combining acetamiprid treatments with MultiPro® guards gave no better protection than the insecticide by itself.
In the middle of the second growing season, for reasons of economy it was unfortunately not possible to reassess all treatments, and so the decision was taken to focus only on those which included acetamiprid (b). Again, the pattern of significant differences in survival was the same at both sites. Mortality due to Hylobius continued to increase, with only around 5–35% of untreated seedlings surviving. Acetamiprid gave between 50 and 60% survival at Wainhope Linn and 30–60% at Binky Burn, although practically all of the remaining trees had suffered some damage, but there were no significant differences in survival between any of the treatments containing acetamiprid. However, there were large numbers of dead trees in the MultiPro® plus acetamiprid treatment that although excluded from the analysis because they were not positively identified as such when assessed in 2015, were almost certainly killed by Hylobius in 2014.
Experiment 5
At Spadeadam Forest, survival after one growing season in the untreated plots (treatment 1) was only around 15%, indicating a very high Hylobius population was present (a). Compared to the untreated controls, pre-treating plants with 0.037 g a.i. stem−1 acetamiprid using knapsack sprayers in the nursery beds (treatment 3) significantly (p < 0.05) improved survival to around 95%. However, for the transplants sprayed using the new nursery pre-treatment machine (treatment 2), where a lower rate of 0.009 g a.i. stem−1 acetamiprid was applied in error, survival was only c. 50%. This was significantly (p < 0.05) better than the untreated control, but significantly worse than the other acetamiprid treatments.
Figure 5. (A) Transplant status after one growing season at Stourcleugh, Spadeadam Forest, in 2016, Experiment 5. Notes: Lettering indicates a statistical difference in the proportion of treatment survival (alive/alive (damaged) versus dead (Hylobius), corrected for multiple comparisons. Other mortality and missing transplants were excluded from the statistical analysis. (B) Transplant status after two growing seasons at Stourcleugh, Spadeadam Forest, in 2017, Experiment 5. Notes: Lettering indicates a statistical difference in the proportion of treatment survival (alive/alive (damaged) versus dead (Hylobius), corrected for multiple comparisons. Other mortality and missing transplants were excluded from the statistical analysis.
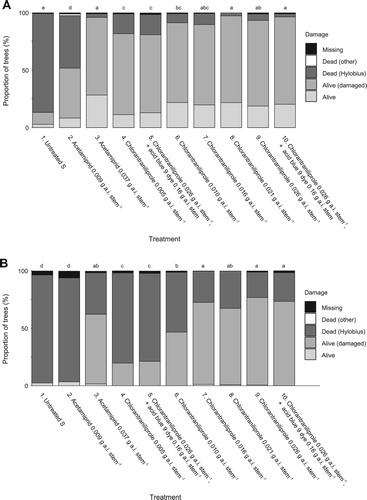
All rates of chlorantraniliprole significantly (p < 0.05) improved protection compared to the untreated control, but rates of 0.016 g a.i. stem−1 or more (treatments 7–9) were most effective, giving the same protection as acetamiprid, with around 95% of trees surviving. The addition of marker dye to the spray mix had no effect on the level of protection provided by chlorantraniliprole (treatments 5 and 10).
In the subsequent year, mortality due to Hylobius browsing continued to increase, and by the end of the second growing season, for the untreated trees, or those where a low rate of acetamiprid had been applied in error, only around 2% had survived (b). Higher application rates of 0.037 g a.i. stem−1 acetamiprid were still providing some protection, allowing survival of around 60%, which was significantly better than the untreated control. All rates of chlorantraniliprole still provided some protection, which was not affected by the addition of a dye marker, but applications of 0.016 g a.i. stem−1 or higher provided the most benefit, a single application before planting allowing around 75% of trees to survive for two years, which was not statistically significantly different (p > 0.05) to the protection provided by acetamiprid. However, almost all trees that survived, whether treated with acetamiprid or chlorantraniliprole, had been browsed to some extent by Hylobius.
Discussion
The experiments reported here provide additional evidence of high efficacy rates of acetamiprid in protecting young trees from attack by Hylobius, without causing phytotoxic effects on plants. As a pre-planting pre-treatment, acetamiprid performed consistently as well as, or better than, the industry standard alpha-cypermethrin applied via the Electrodyn®. Except for Experiment 3, transplants pre-treated with acetamiprid regularly survived the first season on-site, irrespective of whether it was applied on its own, or in combination with physical products (e.g. Kvaae® wax). Acetamiprid is translocated systemically once it is taken up by the plant, but it was not known whether, for dormant trees after they were lifted, uptake might be more effective if roots were treated as well as stems and foliage. However, our work found that no additional benefit in efficacy was gained from applying acetamiprid to roots, so pre-treatment sprays in the future can be limited to targeted sprays of stems and foliage only. We also found that as long as the amount of acetamiprid active ingredient applied to each plant remained the same, lower spray volume rates could be used without affecting efficacy, meaning that for this systemically translocated insecticide, not all the plant necessarily needs to be coated initially with the spray mix to achieve good protection.
The Electrodyn® system allows large quantities of trees to be pre-treated inside factory buildings located at forest nurseries, with a very low risk of operator or environmental contamination. However, a significant disadvantage of the system is that it requires pesticides to be specially formulated with oil and a cyclohexanone solvent carrier, and the only product currently available contains alpha-cypermethrin, a synthetic pyrethroid insecticide the use of which the forest industry was seeking to phase out. Registering a pesticide containing an alternative active ingredient for use in the Electrodyn® system was judged likely to be prohibitively expensive and impractical by Forestry Commission nursery managers. Therefore, a new machine was developed by the Forestry Commission to replace the Electrodyn® for pre-treating high quantities of trees on an operational basis. This consisted of a conveyor belt system passing seedlings through an enclosed booth where aqueous sprays of any approved, conventionally formulated insecticide could be applied at low volume rates (5 ml of diluted pesticide mix per tree) to minimise the amount of time trees need to be left un-bagged for drying. Unfortunately, given the low rate of acetamiprid active ingredient applied in Experiment 5, it was not possible in our work to demonstrate that the new pre-treatment machine could provide the same level of protection from Hylobius browsing as when acetamiprid is applied on a small scale experimental basis using knapsack sprayers. However, subsequent work has demonstrated that when correctly calibrated, the pre-treatment machine can apply acetamiprid such that plants are successfully protected, and the system is now in use operationally in Forestry England and Forestry and Land Scotland nurseries.
A further alternative method of pre-treating trees investigated in our work was by over-spraying nursery seedbeds using a tractor, before the trees were lifted. The idea behind this treatment was that it would be cheaper to apply than spraying individual trees, and would reduce the amount of plant handling required, which itself can damage trees. However, for this method of application, the maximum rate that could be applied to the nursery beds according the approved Gazelle product label (Certis Citation2017) gave an equivalent of only 0.0001 g a.i. stem−1 acetamiprid. Even when a rate that was over sixty times the approved label maximum for seedbed treatment was used, this only achieved an equivalent of 0.006 g a.i. stem−1 acetamiprid. For both seedbed over-spraying treatments the protection offered was less than when treating individual trees, and given approval for a higher rate of seedbed application is very unlikely to be permitted, we conclude that this approach is likely to be impractical on an operational basis and is therefore not worth pursuing further.
Despite the good early protection from Hylobius browsing provided by acetamiprid, where it was monitored, tree survival deteriorated over the second growing season. This indicates that on sites with high Hylobius populations, for trees initially pre-treated with 0.037 g a.i. stem−1 acetamiprid, top-up sprays of 0.037 g a.i. stem−1 acetamiprid are likely to be required in the spring of the second year after planting.
Overall, the results from this study support those from complementary research reported by Willoughby et al. (Citation2020) and Moore et al. (Citationin prep.), and demonstrate that the neonicotinoid insecticide acetamiprid is an effective alternative to synthetic pyrethroids in preventing Hylobius damage to young trees. However, neonicotinoid insecticides have attracted considerable attention in recent years due to their links to pollinator decline, particularly bees, and because of their wider ecosystem impacts (e.g. Lundin et al. Citation2015; Pisa et al. Citation2015). For this reason, many neonicotinoids have been banned for outdoor use in Europe. By contrast acetamiprid is less acutely toxic to bee species, and although it has been demonstrated that it can still have sub-lethal effects (e.g. Pisa et al. Citation2017; Shi et al. Citation2020), it has not to date been linked to bee decline in the same way as other neonicotinoids have, and therefore outdoor use is still permitted in many European countries. In the UK, the Health and Safety Executive, the government agency whose primary role is to ensure human and environmental safety, have granted approval for acetamiprid use in forestry (Health and Safety Executive Citation2012), although all such regulatory decisions are kept under review as new evidence emerges. When used as a pre- or top-up treatment of young trees, once the spray is dry, it is likely that bees would have to ingest parts of the seedling to have any significant exposure to the insecticide. Since they do not feed on trees, and are unlikely to land on them whilst foraging, and as the surrounding soil and vegetation are not sprayed, cross contamination of bees arising from forest use of acetamiprid is unlikely, as long as scrupulous attention is paid to operational best practice (Willoughby et al. Citation2017). Nevertheless, we recommend that acetamiprid should only be used in the UK and Ireland to protect against Hylobius browsing as part of an integrated pest management approach as described earlier, and only when no other effective, safe and economically viable non-chemical methods exist.
Chlorantraniliprole is a non-neonicotinoid synthetic insecticide that has a relatively low mammalian toxicity, is around a hundred times less toxic to aquatic life than alpha-cypermethrin or cypermethrin, and is around ten times less acutely toxic to bees if ingested than acetamiprid (MacBean Citation2012). Although short term sub-lethal effects are possible (e.g. Williams et al. Citation2020), it has not been linked to bee decline in the same way as some neonicotinoids have. We tested chlorantraniliprole at one site over a two-year period (Experiment 5), where it displayed promising rates of efficacy, comparable to acetamiprid, with no obvious phytotoxic effects on plants. Maximum efficacy was achieved at a rate of 0.016 g a.i. stem−1 chlorantraniliprole, and increasing the dose further did not provide any additional benefit. Nevertheless, as with acetamiprid, tree survival deteriorated over the second growing season, and after two years almost all surviving trees had suffered some browsing. This suggests that for trees initially pre-treated with 0.016 g a.i. stem−1 or more of chlorantraniliprole in the nursery, a top-up spray in the forest of up to 0.016 g a.i. stem−1 chlorantraniliprole is likely to be required in the spring of the second year after planting. Hardy et al. (Citation2020) also reported promising levels of protection over two years, similar to that provided by acetamiprid, for 0.013 g a.i. stem−1 chlorantraniliprole, which is the maximum application rate currently permitted for pre-treatment applications in the UK, assuming a standard post-treatment planting density of 2700 stems ha−1 (Health and Safety Executive Citation2019). We recommend that further trials on chlorantraniliprole should be carried out to confirm effective minimum dose rates.
The marker dye Dysol Turquoise® (at a rate giving an equivalent of 0.16 g stem−1 a.i. Acid Blue 9) did not reduce the efficacy of any of the treatments we tested it on, suggesting that it can be used in spray mixes with chlorantraniliprole, to assist in targeting applications, minimising drift, and in reducing the risk of operator contamination in the forest (Brown et al. Citation2003; Willoughby Citation2007). However, currently in the UK although chlorantraniliprole (as Coragen®) has an Extension of Authorisation for Minor Use (Health and Safety Executive Citation2019) which permits it to be used to pre-treat plants in a forest nursery prior to dispatch, no approved product exists that can be used in the forest for top-up sprays after planting.
Of the experiments planted on restock sites that included different sizes of trees, no benefit appeared to be gained from using larger trees. Thorsen et al. (Citation2001) reported that trees with a root collar dimeter of 10 mm or more, when planted on scarified sites in Scandinavia, were able to tolerate insect browsing damage and suffered negligible Hylobius-induced mortality, but the size of trees used in our work was insufficient to test this finding.
Two of the five experiments reported here examined the effect of cold storage and consequent later planting dates on the efficacy of insecticide protection. At Headley Research Enclosure, in the absence of Hylobius pressure, cold storage had no significant effect on transplants treated with Kvaae® wax. Three month’s cold storage, which represents the most extreme test of possible phytotoxic effects, improved the health of transplants treated with alpha-cypermethrin or acetamiprid. Conversely, insecticide treated plants that were not cold stored, or in the case of acetamiprid stored for a shorter duration, did not fare as well as equivalent untreated plants. However, given the consistent lack of phytotoxic effects of these insecticides in non-cold stored plants in other studies (Willoughby et al. Citation2020) and the fact that the longest duration of storage in our work had no negative effect, we believe that Sitka spruce transplants pre-treated with conventional aqueous sprays of alpha-cypermethrin or acetamiprid are very unlikely to suffer effects of phytotoxicity when cold stored, in contrast to previous findings involving trees pre-treated with permethrin via the Electrodyn® (Morgan Citation1999). This may provide additional operational flexibility to nursery growers. However, when cold stored transplants were planted on a restock site with significant Hylobius pressure the pre-treated trees appeared to offer little protection against Hylobius browsing, and alpha-cypermethrin treated trees fared worse than those that were unsprayed. This apparently poor efficacy of alpha-cypermethrin, albeit at a single site, was unexpected and in contrast to previous findings here and elsewhere (Willoughby et al. Citation2020). It is possible that results from both experiments were confounded by the experimental design which meant that the different storage times also had different planting dates.
Dormant, cold stored trees will continue to consume carbohydrate through maintenance respiration, in order to keep cells healthy and produce compounds that protect them from frost (Pallardy Citation2008). During maintenance respiration, some oxygen will be taken up through the lenticels of stems (Sperling et al. Citation2015), and there is also some evidence that there may be translocation of carbohydrates from roots (Loescher et al. Citation1990). These processes might provide a route by which a systemic insecticide such as acetamiprid, applied to the stem and foliage of trees in December, could continue to be taken up and slowly translocated about the plant whilst it is in cold storage, hence increasing the level of protection afforded when they are subsequently planted in the spring. Although no such benefit, or indeed any consistent effects of cold storage or planting date were found in our work, early results from subsequent trials (not reported here) involving similar treatments to Experiment 3, but utilising an improved experimental design with a common planting date, indicate that cold storage often improves the efficacy of acetamiprid treated trees, again without causing phytotoxicity.
The Kvaae® flexible barrier wax system was tested in four experiments, but it provided variable results. In the absence of Hylobius at Headley Research Enclosure, Kvaae® wax was generally found to be non-toxic across a range of trees species. This was true even when up to 100% of the above ground stem / foliage (but not the terminal bud) were treated, in contrast to results from Scandinavia where it has been suggested that treatment of more than 70% of the above ground parts of the plant significantly reduces tree vitality (Norsk Wax Citation2016). The significantly higher mortality we recorded in Experiment 2 in western red cedar plants with 0% wax cover, compared to those that were coated seems anomalous. It has been suggested that the white colour of the Kvaae® wax can help to reflect solar radiation and reduce heat injury to seedlings (Lalik et al. Citation2020), but it seems equally likely that our result was simply an artefact of variable quality of the planting stock used. On the three restock sites where we tested it, which all had high populations of Hylobius, wax coating conferred no useful additional benefit when used in conjunction with insecticides, and when applied by itself, compared to acetamiprid, the wax tended to give poorer protection against Hylobius browsing. Where damage was caused by Hylobius it is likely to have been due to wax flaking off in cold weather to expose bare stem, weevils walking over the wax to access uncoated areas of the plant. Alternatively “bridging” of brash and other on-site woody detritus may have allowed insects to access and feed on untreated areas of the stem, although Petersson et al. (Citation2006) demonstrated that the presence of dead stalks of wavy hair-grass (Deschampsia flexuosa L.) increased browsing damage because it provided a perceived shelter effect from either predators or temperature extremes for the feeding weevils, rather than because it facilitated better physical access to plants. Lalik et al. (Citation2020) reported that Kvaae® wax was as efficient as alpha-cypermethrin in successfully preventing Hylobius browsing damage on Norway spruce, but like their insecticide treatment, was not effective in protecting Douglas fir. The somewhat poorer performance of Kvaae® wax treated trees in our work might be because our experiments took place on typical restock sites rather than in controlled conditions which may have led to accelerated breakdown of wax and allowed “bridging” over brash; and because we used different tree species over a longer time period, and at a greater scale.
Overall, the results of our experiments on Kvaae® wax were slightly better than those reported by Willoughby et al. (Citation2020), and unlike in that work, we observed no obvious signs of phytotoxicity at higher wax coating levels. However, to date there has been only limited operational use of Kvaae® wax in the UK because of the following: variable performance on the highest population pressure sites; anecdotal reports from large-scale user trials of phytotoxicity, lack of protection, and difficulties in separating trees that had stuck together in planting bags; and concerns over how successful top-up spraying, if required in later years, would be on plants with partial and flaking wax coating of stems.
MultiPro® barriers provided adequate protection for vegetatively propagated Sitka spruce on two sites with intermediate to high Hylobius populations in Experiment 4, though not for conventional planting stock. Using the barriers on acetamiprid treated trees did not improve on the protection provided by the insecticide alone. We did not test how much protection the barrier provided in later years, but Willoughby et al. (Citation2020) reported that even on sites where first year protection was adequate, the barrier had failed by the second growing season; and that one explanation for the poorer results compared to those found in Scandinavia (Petersson et al. Citation2004; Eriksson et al. Citation2018) might potentially be because of higher Hylobius population levels in parts of the UK and Ireland. Alternatively, it is possible that the Hylobius population levels on the sites where trials were conducted may have been lower in Scandinavia than in the UK. In contrast, Hardy et al. (Citation2020) reported that on three intermediate to high population sites in the UK, MultiPro® guards gave adequate protection for two years, similar to that provided by a single applications of acetamiprid or chlorantraniliprole, although on two high population sites protection was inadequate.
Where failure of the MultiPro® barriers occurred in our work, it is likely to have been due to insects accessing the tree stems via gaps left between the base of the sleeves and the stem, or “bridging” caused by brash or vegetation providing access to the upper parts of the transplants where they emerge from the protective barriers. Other problems reported with some types of physical barrier sleeves are that they are difficult and time consuming to fit, particularly over larger plants with bigger branches; it is difficult to bury the lower part of the guards on stony or very friable soil correctly; guards can become dislodged and fail on exposed sites, which are common in upland forestry in the UK; they are between 3 and 20 times more costly than insecticide pre-treatment; and they may make subsequent top-up spraying, if required, prohibitively expensive to carry out (Hardy et al. Citation2020; Willoughby et al. Citation2020).
From the evidence gained in the experiments reported here, we agree with Willoughby et al. (Citation2020) that physical barriers such as MultiPro®, and to a lesser extent Kvaae® wax, may have a role in UK forestry in preventing unacceptable levels of Hylobius damage to newly planted trees, but only if the Hylobius Management Support System (Moore Citation2018) or an alternative, reliable method of estimating population levels is used, and the onsite population is predicted to be low (i.e. < 25 per cent untreated tree loss). Even then, MultiPro® barriers should only be used on sheltered sites, and need to be combined with the use of good-quality planting stock possessing large root collar diameters, an appropriate balance between the dry weight of their roots and shoots (Morgan Citation1999) and with few side branches, which may limit their use in practice to vegetatively propagated material. Suitable site preparation to create a weed and brash free site around the planted tree (Paterson and Mason Citation1999; Willoughby et al. Citation2004) is also essential. In these circumstances, physical barriers, as part of an integrated approach, may be worth considering as a potentially suitable, if more expensive, substitute for the use of insecticides in the UK and Ireland (Willoughby et al. Citation2017). We also recommend that the Conniflex® sand and glue system for treating cell grown planting stock (Nordlander et al. Citation2009), that is used extensively in Sweden, is subject to further test in UK and Irish conditions to determine if it might prove to be a more effective physical barrier than those so far examined.
Conclusions
The experiments reported here confirm the efficacy of acetamiprid as part of the integrated pest management of Hylobius, supporting evidence from elsewhere (Hardy et al. Citation2020; Willoughby et al. Citation2020; Moore et al. Citationin prep). Our results indicate that pre-treating trees with 0.037 g a.i. stem−1 acetamiprid provides a similar if not better level of protection than alpha-cypermethrin or cypermethrin over the first year after planting, but that top-up sprays of 0.037 g a.i. stem−1 acetamiprid are likely to be required in the spring of the second growing season. Trees treated with acetamiprid can be cold stored without any risk of phytotoxicity.
We also found that pre-treating trees with 0.016 g a.i. stem−1 chlorantraniliprole can be equally as effective as acetamiprid in protecting trees, and that dye markers can be safely used to help target spays. However, further trials are required to confirm a minimum effective dose rate for chlorantraniliprole.
Our testing of physical barriers such as MultiPro®, and to a lesser extent Kvaae® wax, lead us to conclude that they may have a role in UK forestry in preventing unacceptable levels of Hylobius damage to newly planted trees, but only as part of an integrated approach where on-site populations of Hylobius are predicted to be low, and where the significant additional costs of using them can be borne. Even then, MultiPro® guards should only be utilised on sheltered, cultivated and weeded sites with a soil texture that permits correct installation, and in combination with well-balanced planting stock with few side branches, which may limit their use in practice to vegetatively propagated material.
Acknowledgements
We are grateful to a wide range of people who have contributed to this work. Forestry England provided four of the experiment sites. Alex Steepe of Delamere nursery, Forestry England, arranged plant supply, cold storage, application of some of the treatments and REL testing; Liz Richardson, Mark Oram, Emyr Algieri, Sue Bellis, Lee Cooper, Steve Coventry, Justin Hardy, Kate Harvey, Trish Jackson, Ian Keywood, John Lakey, David Lloyd, Joe McMinn, Jim Page, Nicola Rivett, Paul Turner and Steve Whall in the Forest Research Technical Services Unit established treated and assessed the experiments. Ed Wilson and Tom Connolly helped with analysing results, Glenn Brearley assisted with producing the Figures, and Helen McKay and two anonymous referees provided helpful comments on early versions of the manuscript. Bruce Sewell (Forestry and Land Scotland), Stephen Smith (Forestry England), David Cross (Natural Resources Wales), Martin Price (Forestry and Land Scotland) and John Morgan (Forestry Commission GB) provided support for this work and advice on operational practices. Advice on experimental treatments was also received from Jarl Markus Pettersen (Norsk Wax).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Björklund N, Nordlander G, Bylund H. 2003. Host-plant acceptance on mineral soil and humus by the pine weevil Hylobius abietis (L.). Agric For Entomol. 5:61–66. doi:10.1046/j.1461-9563.2003.00163.x.
- Brown A, Willoughby I, Clay DV, Moore R, Dixon FL. 2003. The use of dye markers as a potential method of reducing pesticide use. Forestry. 76(4):371–384.
- Certis. 2017. Gazelle SG product label. www.certiseurope.co.uk.
- Eidmann HH. 1985. Silviculture and insect problems. Zeitschrift fur Angewandte Entomologie. 99:105–112.
- Eriksson S, Wallertz K, Karlsson A-B. 2018. Test av mekaniska plantskyddmot snytbaggar i omarkberedd ochmarkberedd mark, anlagt våren 2015. Sveriges Lantbruksuniversitet Rapport 16, 22 pp. https://pub.epsilon.slu.se/15698/1/eriksson_s_et_al_181010.pdf.
- European Commission. 2013. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Official Journal of the European Union L 226. www.ec.europa.eu.
- Forestry Commission. 2019. Forestry statistics 2019. Chapter 1: woodland area and planting. https://www.forestresearch.gov.uk/documents/7722/Ch1_Woodland_FS2019_eFXlcXK.pdf.
- Fox J, Weisberg S. 2011. An R companion to applied regression. 2nd ed. Thousand Oaks, CA: Sage. http://socserv.socsci.mcmaster.ca/jfox/Books/Companion.
- FSC. 2019. FSC Pesticides Policy. FSC-POL-30-001 V3-0 EN. FSC, Bonn. https://fsc.org/en/document-centre/documents/resource/208.
- Hardy C, Sayyed I, Dunbar Leslie A, Dittrich ADK. 2020. Effectiveness of insecticides, physical barriers and size of planting stock against damage by the pine weevil (Hylobius abietis). Crop Prot. 137:105307.
- Harvey CD, Williams CD, Dillon AB, Griffin CT. 2016. Inundative pest control: how risky is it? A case study using entomopathogenic nematodes in a forest ecosystem. For Ecol Manag. 380:242–251.
- Health and Safety Executive. 2012. Extension of authorisation for a minor use of the plant protection product Gazelle SG. Extension of authorisation number 1068 of 2012. www.hse.gov.uk/pesticides.
- Health and Safety Executive. 2019. Extension of authorisation for a minor use of the plant protection product Coragen. Extension of authorisation number 2003 of 2019. www.hse.gov.uk/pesticides.
- Heritage S, Collins SA, Evans HF. 1989. A survey of damage by Hylobius abietis and Hylastes spp. in Britain. In: Proceedings of the Eighteenth IUFRO International Conference of Entomology: Insects affecting Reforestation: Biology and damage. p. 36–42.
- Heritage S, Johnson D, Jennings T. 1997. The use of Permethrin 12ED through the Electrodyn sprayer conveyor to protect forest plants from Hylobius damage. For Comm Res Info Note. 271:1–4.
- Hollis D, Mccarthy M. 2013. UKCP09: Met Office gridded and regional land surface climate observation datasets. Centre for Environmental Data Analysis. www.ceda.ac.uk.
- Houston Durrant T, Mauri A, de Rigo D, Caudullo G. 2016. Picea sitchensis in Europe: distribution, habitat, usage and threats. In: San-Miguel-Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A, editors. European Atlas of forest tree species. Luxembourg: Publ. Off. EU; p. 118–119.
- Kindvall O, Nordlander G, Nordenhem H. 2000. Movement behaviour of the pine weevil Hylobius abietis in relation to soil type: an arena experiment. Entomol Exp Appl. 95:53–61. doi:10.1046/j.1570-7458.2000.00641.x.
- Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. Lmertest package: tests in linear mixed effects models. J Stat Softw. 82(13):1–26. doi:10.18637/jss.v082.i13.
- Lalik M, Galko J, Nikolov C, Rell S, Kunca A, Modlinger R, Holuša J. 2020. Non-pesticide alternatives for reducing feeding damage caused by the large pine weevil (Hylobius abietis L.). Ann Appl Biol. 177:132–142. doi:10.1111/aab.12594.
- Långström B, Day KR. 2007. Damage, control and management of weevil pests, especially Hylobius abietis. In: Lieutier F, Day KR, Battisti A, Grégoire J-C, Evans HF, editors. Bark and wood boring insects in living trees in Europe, a synthesis. Dordrecht: Springer; p. 415–444.
- Leather SR, Day KR, Salisbury AN. 1999. The biology and ecology of the large pine weevil, Hylobius abietis (Coleoptera: Curculionidae): a problem of dispersal? Bull Entomol Res. 89:3–16.
- Lenth R, Singmann H, Love J, Buerkner P, Herve M. 2019. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.3.3. https://CRAN.R-project.org/package=emmeans.
- Loescher WH, McCamant T, Keller JD. 1990. Carbohydrate reserves, translocation, and storage in woody plant roots. HortScience. 25(3):274–281.
- Lundin O, Rundlöf M, Smith HG, Fries I, Bommarco R. 2015. Neonicotinoid insecticides and their impact on bees: a systematic review of research approaches and identification of knowledge gaps. PLoS One. 10:e0136928. doi:10.1371/journal.pone.0136928.
- MacBean C, editor. 2012. The pesticide manual: a world compendium. 16th ed. Alton: British Crop Protection Council.
- McKay HM. 1992. Electrolyte leakage from fine roots of conifer seedlings: a rapid index of plant vitality following cold storage. Can J For Res. 22(9):1371–1377.
- McKay HM, Aldhous JR, Mason WL. 1994. Lifting, storage, handling and dispatch. In: Aldhous JR, Mason WL, editors. Forest nursery practice. Forestry Commission Bulletin 111. London: HMSO; p. 198–222.
- Moore R. 2004. Managing the threat to restocking posed by the large pine weevil, Hylobius abietis: the importance of time of felling of spruce stands. Forestry Commission Information Note 61. Forestry Commission, Edinburgh.
- Moore R. 2018. Hylobius Management Support System (MSS). https://www.forestresearch.gov.uk/tools-and-resources/tree-health-and-protection-services/hylobius-management-support-system/.
- Moore R, Brixey JM, Milner AD. 2004. Effect of time of year on the development of immature stages of the large pine weevil (Hylobius abietis L.) in stumps of Sitka spruce (Picea sitchensis Carr.) and influence of felling date on their growth, density and distribution. J Appl Entomol. 128:167–176.
- Moore R, Wilson E, Willoughby IH, Connolly T, Sayyed I, Leslie K, Straw N. in prep. The insecticides acetamiprid and imidacloprid can effectively protect Sitka spruce transplants from damage by the large pine weevil Hylobius abietis.
- Morgan JL. 1999. Forest tree seedlings – best practice in supply, treatment and planting. Forestry Commission Bulletin 121. Forestry Commission, Edinburgh.
- Nordlander G, Nordenhem H, Hellqvist C. 2009. A flexible sand coating (Conniflex) for the protection of conifer seedlings against damage by the large pine weevil Hylobius abietis. Agric For Entomol. 11:91–100.
- Norsk Wax. 2016. Substitution of insecticides with wax. http://www.metla.fi/tapahtumat/2016/taimitarhapaivat/Pettersen.pdf.
- Olenici N, Manea IA, Olenici V, Tomescu R. 2014. Efficacy of conifer seedling protection against pine weevil damage using neonicotinoids and metaflumizone insecticides. Bull Transylvania Univ Brasov, II, For Wood Ind Agric Food Eng. 7:29–36.
- Örlander G, Nilsson U. 1999. Effect of reforestation methods on pine weevil (Hylobius abietis) damage and seedling survival. Scand J For Res. 14:341–354. doi:10.1080/02827589950152665.
- Pallardy SG. 2008. Physiology of woody plants. 3rd ed. London: Elsevier.
- Paterson DB, Mason WL. 1999. Cultivation of soils for forestry. Forestry Commission Bulletin 119. Forestry Commission, Edinburgh.
- Petersson M, Nordlander G, Örlander G. 2006. Why vegetation increases pine weevil damage: bridge or shelter? For Ecol Manag. 225:368–377. doi:10.1016/j.foreco.2006.01.012.
- Petersson M, Örlander G. 2003. Effectiveness of combinations of shelterwood, scarification, and feeding barriers to reduce pine weevil damage. Can J For Res. 33:64–73.
- Petersson M, Örlander G, Nilsson U. 2004. Feeding barriers to reduce damage by pine weevil (Hylobius abietis). Scand J Forest Res. 19:48–59.
- Petersson M, Örlander G, Nordlander G. 2005. Soil features affecting damage to conifer seedlings by the pine weevil Hylobius abietis. Forestry. 78(1):83–92. doi:10.1093/forestry/cpi008.
- Pisa LW, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Downs CA, Goulson D, Kreutzweiser DP, Krupke C, Liess M, McField M, et al. 2015. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ Sci Pollut Res. 22:68–102.
- Pisa L, Goulson D, Yang EC, Gibbons D, Sánchez-Bayo F, Mitchell E, Aebi A, van der Sluijs J, MacQuarrie CJ, Giorio C, et al. 2017. An update of the worldwide integrated assessment (WIA) on systemic insecticides. Part 2: impacts on organisms and ecosystems. Environ Sci Pollut Res. doi:10.1007/s11356-017-0341-3.
- R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Shi J, Yanga H, Yua L, Liao C, Liua Y, Jin M, Yana W, BoWua X. 2020. Sublethal acetamiprid doses negatively affect the lifespans and foraging behaviours of honey bee (Apis mellifera L.) workers. Sci Total Environ. 738. doi:10.1016/j.scitotenv.2020.139924.
- Sperling O, Earles JM, Secchi F, Godfrey J, Zwieniecki MA. 2015. Frost induces respiration and accelerates carbon depletion in trees. PLoS One. 10(12):e0144124. doi:10.1371/journal.pone.0144124.
- Thorsen AA, Mattsson S, Weslien J. 2001. Influence of stem diameter on the survival and growth of containerized Norway spruce seedlings attacked by pine weevils (Hylobius spp.). Scand J For Res. 16(1):54–66.
- UKWAS. 2018. The UK woodland assurance standard: fourth edition, version 4. UKWAS, Edinburgh. www.ukwas.org.uk.
- Williams JR, Swale DR, Anderson TD. 2020. Comparative effects of technical-grade and formulated chlorantraniliprole to the survivorship and locomotor activity of the honey bee, Apis mellifera (L.). Pest Manage Sci. 76(8):2582–2588. doi:10.1002/ps.5832.
- Willoughby I. 2007. Using dye markers to reduce pesticide use. Forestry Commission Technical Note 16. Forestry Commission, Edinburgh.
- Willoughby I, Evans H, Gibbs J, Pepper H, Gregory S, Dewar J, Pratt J, McKay H. 2004. Reducing pesticide use in forestry. Forestry Commission Practice Guide 15. Forestry Commission, Edinburgh, 140 pp.
- Willoughby IH, Moore R, Moffat AJ, Forster J, Sayyed I, Leslie K. 2020. Are there viable alternatives to the use of conventional insecticides for the protection of young trees from damage by the large pine weevil Hylobius abietis L. in UK forestry? Forestry. 93(5):694–712. https://academic.oup.com/forestry/advance-articlepdf/doi/10.1093/forestry/cpaa013/33337732/cpaa013.pdf.
- Willoughby I, Moore R, Nisbet T. 2017. Interim guidance on the integrated management of Hylobius abietis in UK forestry. Forest Research Note, 28 pp. https://www.forestresearch.gov.uk/documents/607/FR_InterimguidanceonmanagementHylobiusabietis_2017.pdf.
