Abstract
The current study was designed to compare the radiosensitizing effects of oxaliplatin and 5-fluorouracil (5FU) in a human colon cancer cell line. A human colon cancer cell line (S1) was treated with various doses of oxaliplatin, 5FU, radiation, and combinations thereof. Various clinically used schedules were mimicked. 5FU was either incubated during 1 h (“bolus”) or 24 h (“continuous infusion”). When combining oxaliplatin and 5FU, an isobologram analysis revealed synergistic effects, regardless of 5FU schedule. The IC10 and IC50-doses for the drugs where then combined with radiotherapy. With equitoxic drug doses (IC50), radiosensitization was observed in the following order: oxaliplatin > 5FU 24 h > 5FU 1 h exposure. The degree of potentiation corresponded to approximately 0.8 Gy, 0.7 Gy, and 0.2 Gy, respectively. In this experimental setting, oxaliplatin seemed to be a better radiosensitizer than 5FU, and longer incubation time with 5FU was better than short exposure.
Colorectal cancer is one of the most common malignancies in western countries Citation[1]. In resectable cases, the mainstay of treatment is surgery. In around 10% of new cancers, the tumour is locally advanced and unresectable. These tumours are usually treated with long-term radiotherapy with the purpose of shrinking the tumour and facilitating surgery Citation[2]. In order to potentiate the effect, radiotherapy is often combined with chemotherapy. The most common drug in colorectal cancer is 5-fluorouracil (5FU). 5FU has limited anti-tumour effect as a single drug and is usually combined with leucovorin (LV), which stabilizes the complex between the active metabolite FdUMP and thymidylate synthase Citation[3], which is one of the targets of the drug. 5FU also has other mechanisms of action, such as incorporation into DNA and RNA. The impact of these various modes of action depends on how the drugs are administered; as a rapid bolus injection or as a protracted intravenous infusion for one or several days Citation[4].
In recent years several new agents with activity against colorectal cancer have been introduced Citation[5–7]. One of those is oxaliplatin. When added to infusional 5FU/LV in patients with metastatic colorectal cancer, oxaliplatin has been shown to significantly increase response rates and time to disease progression, compared with 5FU/LV alone Citation[8], Citation[9]. In a Nordic study, bolus 5FU/LV was used in combination with oxaliplatin, also yielding high response rates Citation[10].
Oxaliplatin is a platinum-based compound which has similar mechanisms of action as cisplatin Citation[11], mediated through formation of DNA adducts. Cisplatin has been shown to potentiate radiotherapy Citation[12], Citation[13] and there are reasons to believe that also oxaliplatin could increase the effect of radiation. Indeed, preclinical studies have suggested that this is the case Citation[14], Citation[15].
Early clinical studies have shown promising results when oxaliplatin and 5FU were combined with long-term radiation in locally advanced rectal cancer, in terms of response rate, down-staging, and tolerance Citation[16]. In this, and in most other chemoradiation studies, 5FU has been administered as a continuous infusion. However, bolus 5FU/LV has also been used in combination with oxaliplatin and radiotherapy Citation[17], with high response rates. No comparisons have been made between bolus and infusional 5FU in combination with radiotherapy.
Thus, there are indications that oxaliplatin might be a useful radiosensitizer. However, the degree of sensitization, as compared with that of 5FU, is unknown. It is also unclear which of the 5FU dosing schedules, bolus or continuous infusion, provides the best potentiation of radiation. This study was carried out to elucidate the interactions between radiation, 5FU and oxaliplatin in a colon cancer cell line. The primary aim of this study was to compare the anti-tumoural effects of oxaliplatin and 5FU in combination with radiotherapy. Another purpose was to investigate the radiosensitizing effects of short-term and long-term exposure of 5FU, thus mimicking bolus and infusional therapy, respectively. In the radiotherapy experiments, equitoxic doses of the respective drugs were used. Therefore, the first part of this study was designed to determine the cyotoxic effect and interactions of the drugs alone.
Materials and methods
Drugs and chemicals
Oxaliplatin was obtained from Sanofi-Synthelabo (Eloxatin®) and was dissolved in water before use, and 5-fluorouracil was obtained as solution from Nycomed (Fluracedyl®, Nycomed AB, Stockholm).
Cell lines and growth conditions
A human colon cancer cell line, S1 (derived from the S1 clone of LS-180 colon carcinoma cells Citation[18]) was used. The cells were maintained in logarithmic growth as monolayer cultures in D-MEM medium (Gibco) supplemented with 10% heat-inactivated fetal calf serum and antibiotic (Gibco) (100 units/ml penicillin and 100 units/ml streptomycin sulphate), under a humidified 5% CO2 atmosphere at 37°C.
Design of experiment 1 (chemotherapy only)
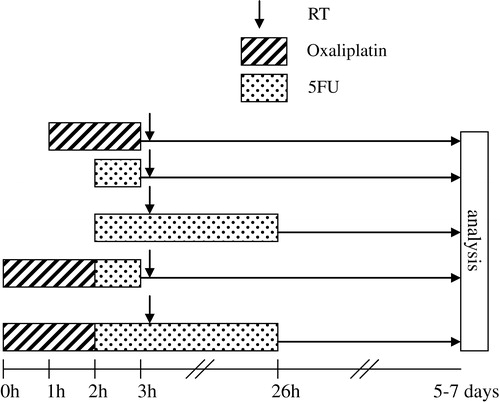
In the first set of experiments, cytotoxicity of the drugs alone was examined. The treatment schemes were chosen to imitate various clinical schedules (). When patients receive oxaliplatin it is almost always administered as a 2h-infusion. Therefore a 2 h-exposure for oxaliplatin was chosen in our study. Regarding 5FU, clinical pharmacokinetical studies after an intravenous bolus injection have shown an initial plasma half-life ranging from 5 to 20 minutes Citation[4], followed by protracted late elimination phase. Based on this we chose an exposure time of 1 h to mimic bolus 5FU. Continuous infusion of 5FU for treatment of patients with colorectal cancer is usually administered for 24 or 48 h. In this study an exposure time of 24 h was selected. Two various schedules were used when combining the two drugs. Oxaliplatin plus bolus 5FU corresponds to Nordic FLOX Citation[10], whereas oxaliplatin combined with 24 h exposure of 5FU resembles clinically used schedules with infusional 5FU Citation[9], Citation[19]. In all of the clinically used treatment schemes, LV is also included. Since LV is merely used to potentiate 5FU, without adding any new mechanism of action, similar cytotoxic effect can be achieved by increasing the dose of 5FU. For this reason and to reduce the number of variables, our experiments were performed without LV.
From these chemotherapy experiments, we calculated the IC10 and IC50 values, i.e. the concentrations of drug causing a 10 or 50% reduction of cell growth, respectively, compared with untreated cells. The results from combined treatments were plotted in isobolograms in order to investigate levels of synergy, see details below.
Design of experiment 2 (chemoradiation)
In the second set of experiments, cells were pretreated with IC10 and IC50 doses of oxaliplatin and/or 5FU (1 h or 24 h exposure), followed by radiotherapy. The chemotherapy time schedules were identical as in experiment 1 (). Radiotherapy was delivered as a single treatment. To the cells treated with oxaliplatin and/or 1 h 5FU, irradiation was delivered at 1 h after removal of the drug-containing medium. This 1 h-interval is derived from a schedule used in a recently completed Nordic study on chemoradiation in locally advanced rectal cancer. Cells treated for 24 h with 5FU, were radiated at 1 h after the beginning of the 5FU exposure.
Treatment experiment 1
Cells (1000 cells/well) were seeded in 96 well plates, and allowed to adhere for 48 h before addition of drug. Oxaliplatin was added to the medium and cells were incubated for 2 h at concentrations ranging from 0 to 8 µM. As for 5FU, cells were exposed during 1 h at concentrations ranging from 0 to 80 µg/mL or 24 h at concentrations ranging from 0 to 10 µg/mL. In the combination experiments, cells in 96 well plates were treated with oxaliplatin during 2 h. Thereafter, the drug containing medium was removed, and cells were exposed for 1 h or 24 h to 5FU. After drug removal, cells were allowed to regrow in drug-free medium for 5 days before the cell survival was determined with MTT and the drug interactions were analysed by means of isobologram plotting, see below. Experiment 1 was performed in duplicate or triplicate.
Treatment experiment 2
To determine the cytotoxic effects of radiation, and combination of drugs with radiation, cells were grown in 25 cm2 flasks. After a 2 h exposure to oxaliplatin, or a 1 h exposure to 5FU, or combinations thereof, the drug was removed and the cells were counted and transferred to 15 ml tubes before exposure to single radiation doses ranging from 0 to 4 Gy. Irradiation was delivered with a Gammacell 2000 (MØlsgaard medical Denmark). After irradiation, cells were seeded in 96 well plates at different cell densities in order to maintain exponential growth. Seven days later, the cytotoxic effect was determined by MTT assay. When cells were pretreated with 5FU for 24 h alone or in combination with oxaliplatin, cells were exposed for 1 h to 5FU, counted and transferred to 15 ml tubes before irradiation in the presence of 5FU. Cells were then seeded in 96 well plates, and after a total exposure time of 24 h the 5FU-containing medium was removed and replaced by drug-free medium. Seven days later, the cytotoxic effect was determined using the MTT assay. Due to differences in the cell culturing conditions, the results from experiments 1 and 2 were not totally comparable. Therefore, isobologram analysis of synergy between irradiation and chemotherapy could not be performed. Experiment 2 was performed in duplicate.
MTT assay
Cell survival was analysed by the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay Citation[20] (MP Biomedicals Inc., Germany). Briefly, the tetrazolium salt was added to each well in the 96 well plates. During a 3 h exposure of the cells to MTT water-insoluble, dark blue formazan crystals are formed due to the action of the mitochondrial enzyme succinate-dehydrogenase. Thereafter, sodium dodecyl sulphate, SDS, was added to each well in order to solubilize the formazan crystals. The optical density of the dissolved material is then measured spectrophotometrically, yielding absorbance, which directly correlates to the number of metabolically active cells in the culture.
Test for synergy, experiment 1
For each combination the IC50 values were calculated, and the data from these experiments were analysed using an isobologram method Citation[21]. In brief, the IC50-isobolgram shows the doses required to inhibit cell growth by 50% after exposure to oxaliplatin alone, 5FU alone, or combinations thereof. The area between the two lines in and indicates the “envelope of additivity”. The results from each cell survival curve in experiment 1 were merged and plotted. Dots located to the left, within, or to the right of the “envelope of additivity” indicate synergy, additivity and antagonism, respectively, between the two drugs.
Statistics
Experiments were performed in at least duplicates. For each of the series, a linear regression model of log10 cell survival on dose was fitted using data for five doses with survival surrounding the corresponding IC50 value. In this model the IC50-value can be written as a function of the slope b and the intercept a: IC50=(log10(50)-a)/b. We estimated the IC50-value for each series by plugging in the estimates of the slope and intercept parameters. The variance of each IC50-estimate was approximated using a second order Taylor expansion of the above equation, so called Gauss approximation. Z-tests were used to test null hypotheses of equal IC50-values in two independent series, see . The Bonferroni method was used to adjust for multiple comparisons.
Results
Cytotoxic effect of the drugs only
Increasing concentrations of oxaliplatin or 5FU reduced the viability of the cells. The IC10 and IC50 values, summarized in , indicate that the concentration of 5FU needed to obtain the same level of cytotoxicity was approximately 6 to 14 times lower when the cells were exposed to the drug for 24 h compared with 1 h. When increasing concentrations of 5FU was combined with oxaliplatin, cell viability decreased compared with oxaliplatin treatment alone, as illustrated in . We found that the IC50 values obtained from the drug combinations to be within or to the left of the envelope of additivity in the isobolograms, indicating additive to synergistic effects. Similar effects were observed for a short (1 h) or a long (24 h) 5FU exposure time, as illustrated in and .
Figure 2. Survival of S1 cells exposed to varying concentrations of oxaliplatin alone (□), or in combination with different concentrations of 5FU: (A) 1 h to 10 (▪), 20 (▴), or 30 (![]()
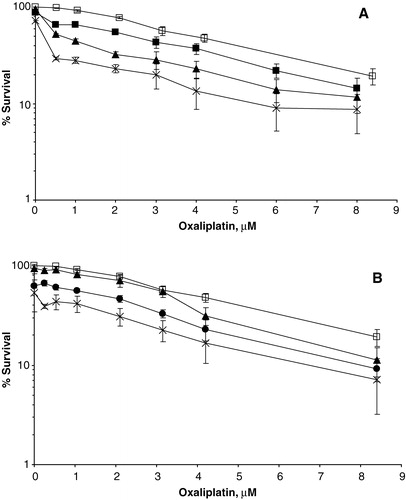
Figure 3. Isobolographic analysis of oxaliplatin combined with 5FU (1 h exposure) for S1 cells at 50% survival. Dots to the left of the envelope of additivity (demarcated by the two lines) indicate synergistic effects between the drugs.
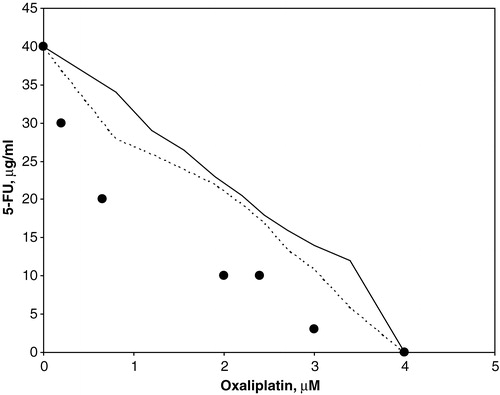
Figure 4. Isobolographic analysis of oxaliplatin combined with 5FU (24 h exposure) for S1 cells at 50% survival. Dots to the left or within the envelope of additivity (demarcated by the two lines) indicate synergistic and additive effects between the drugs, respectively.
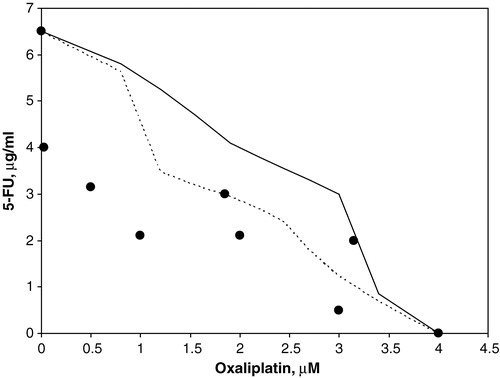
Table I. Effect of oxaliplatin and 5FU alone on cell survival of S1 human colon cancer cells. Values are reported in µM for oxaliplatin, and µg/ml for 5FU.*
Combined oxaliplatin/5FU/irradiation treatments
shows the cell survival as a function of radiation dose, with or without pre-treatment with the IC50 dose of each drug. These curves were normalized to 100%, thus subtracting the pure cytostatic effect.
Figure 5. Cell survival of S1 cells treated with radiation alone (□) or in combination with oxaliplatin (•), 5FU 1 h (▪), or 5FU 24 h (▴) at their IC50 doses. Normalized to 100%. Bars indicate SEM.
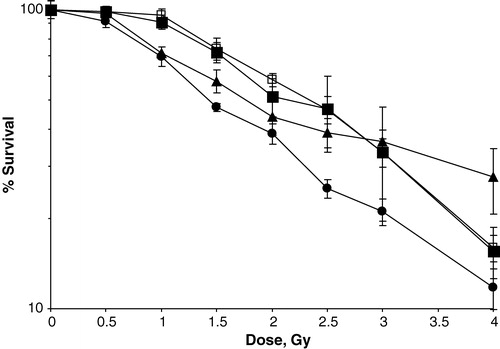
Similar plots were constructed for all chemoradiation combinations and the radiation dose needed to obtain a 50% inhibition of cell growth is summarized in . Apart from the 1 h exposure of 5FU, all drugs and combinations resulted in a significant enhancement of the cytotoxic effect, compared with radiation alone. Both oxaliplatin and 24 h treatment with 5FU were found to potentiate radiation significantly better than 1 h exposure of 5FU (p < 0.001 and p = 0.02), at equitoxic drug doses. The difference between oxaliplatin and 5FU 24 h was not statistically significant (p = 0.2) in the IC50-range, but at radiation doses of > 2 Gy a gradually increasing separation of the two curves was observed (), indicating superior radiopotentiation of oxaliplatin compared with 24 h exposure of 5FU. At higher radiation doses however, care should be taken to evaluate the radiopotentiating effect of the drugs and combinations, due to the limitation of the biological assay used as discussed below. When both oxaliplatin and 5FU were combined with radiation, a further enhancement of the cytotoxic effect was seen, most notably after oxaliplatin plus 24 h exposure of 5FU (). The enhancement of the cytotoxic effect was significant for all combinations except when comparing the IC10 values of the combination of oxaliplatin and 5FU 24 h with 5FU 24 h.
Figure 6. Cell survival of S1 cells treated with radiation alone (□) or combined with: oxaliplatin IC10+5FU 1 h IC10 (▪), oxaliplatin IC10+5FU 24 h IC10 (▴), oxaliplatin IC50+5FU 1 h IC50 (•), or oxaliplatin IC50+5FU 24 h IC50 (![]()
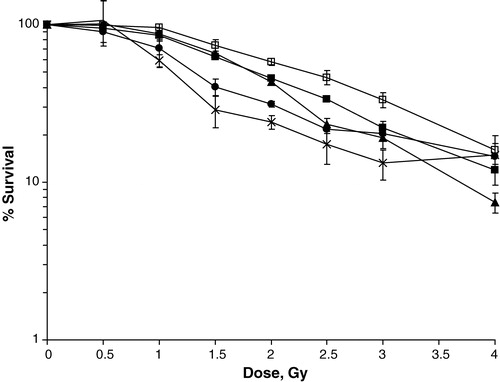
Table II. Radiation dose needed for 50% cell kill (IC50) alone, or in combination with 5FU or oxaliplatin at their individual IC50 or IC10 values or combination thereof.*
Discussion
Oxaliplatin in combination with 5FU/LV has in recent years become one of the standard treatments for metastatic colorectal cancer. In these combinations, 5FU/LV can be administered either as a bolus injection or protracted intravenous infusion. Locally advanced tumours located in the rectum are often subjected to preoperative radiotherapy combined with concomitant 5FU-based chemotherapy. There are several preclinical and early clinical studies suggesting that also oxaliplatin could have radiosensitizing properties Citation[14–16]. However, the degree of radiosensitization by oxaliplatin in comparison with 5FU is yet unknown. It is also unclear whether bolus or continuous infusion of 5FU/LV provides the best enhancement of radiation. The purpose of this study was to elucidate these issues by mimicking various clinical schedules in a colon cancer cell line. One can discuss the relevance of cell line studies vis-à-vis the clinical situation, given the obvious differences in tumour growth conditions, blood supply, host reactions etc. However, we still feel that these kinds of widely used in vitro models can provide valuable information of comparative cytotoxicity on the cellular level.
In the first part of this study, the cytotoxicity of oxaliplatin and 5FU, without radiation, was investigated. A clear synergistic effect was observed when the two drugs were combined, regardless of the 5FU schedule, which confirms previous studies Citation[22], Citation[23]. Several various mechanisms for this interaction have been suggested. Oxaliplatin may stabilize the 5FdUMP-TS binding by reducing the folate pool or it may interfere with 5FU incorporation into RNA Citation[22]. Conversely, 5FU has been shown to inhibit repair of DNA cross-links caused by cisplatin Citation[24]. In view of the functional similarities between these two platinum agents, it is possible that 5FU may decrease DNA damage repair also in combination with oxaliplatin.
In the chemoradiation experiments, equitoxic doses of the two drugs were combined with radiation. Due to differences in cell culturing conditions in the two experiments, the isobologram technique could not be applied to determine whether the effects of chemotherapy plus radiation were additive, synergistic or antagonistic. Subsequently, the conclusions that can be drawn from the chemoradiation experiment were restricted to relative comparisons between the radiopotentiating effects of the different doses and combinations of chemotherapy, which, however, was sufficient for the purpose of this study.
The addition of oxaliplatin or 5FU led to decreased cell survival in combination with irradiation. It must be noted that the curves in and are normalized to 100%. This means that the pure cytostatic effect has been subtracted and differences between curves reflect the radiosensitization achieved by adding the drug to radiation. After addition of single drugs at their IC50 dose to radiation, the radiopotentiating effect was observed in the following order: oxaliplatin > 5FU 24 h exposure > 5FU 1 h exposure. The order of magnitude of potentiation can be extracted from . With single drugs at their IC50 dose, the addition of oxaliplatin, 5FU 24 h and 5FU 1 h corresponded to approximately 0.8 Gy, 0.7 Gy and 0.2 Gy respectively. These effects were observed at IC50 dose of radiation, which was around 2 Gy, i.e. in the clinically most relevant dose range. To our knowledge, this is the first study suggesting that oxaliplatin may be a better radiosensitizer than 5FU. Our finding that oxaliplatin potentiates radiotherapy confirms previous studies in cell lines and animal models Citation[14], Citation[15]. Schedule dependence for radiosensitization of 5FU has also been reported in a previous study Citation[25], in which the radiopotentiating effect was higher with continuous 5FU exposure as compared with short-term 5FU treatment of a pancreatic cancer cell line. Adding both oxaliplatin and 5FU to radiation significantly enhanced the radiopotentiating effect as compared with addition of oxaliplatin or 5FU alone. In most cases, the radiopotentiating effect of the combinations corresponded to an additive or near-additive effect. The one exception is for the IC10 combination with 5FU 24h exposure. In this case, the effect of the combination was less than what one would expect from an additive effect of both drugs alone. The reason for this is unclear. The mechanism for oxaliplatin′s radiosensitization is unclear. It may be that the platinum adducts prevent effective repair of the DNA damage caused by irradiation. Effects on the cell cycle progression could be another possible mechanism. Oxaliplatin has been shown to cause an arrest in the G2/M phase Citation[26], Citation[27], during which cells are most vulnerable for irradiation. Further studies are needed to clarify this issue.
In the present study, cell survival was evaluated with MTT, since the S1 cell line does not form colonies. MTT is a well-established method for studies on chemotherapeutic agents. For irradiation experiments, MTT has been shown less sensitive than clonogenic assays, since it tends to over-estimate cell survival Citation[28]. However, a study by Banasiak et al. Citation[28] showed similar ranking order of radiosensitivity between various cell lines, when cell survival was assessed with clonogenic and MTT assays and they conclude that MTT is acceptable to use for cell lines will low plating efficiencies. To further illuminate this problem, we performed a separate experiment on colony forming V79 hamster fibroblast cells that were treated with oxaliplatin, 5FU and irradiation. Cell survival was analysed with both colony forming assay and MTT. We found a slight overestimation of cell survival by MTT compared with the colony-forming assay, especially after high radiation doses, but the relative ranking order of radiosensitization with different drug combinations was very similar, regardless of cell survival estimation method. It was of interest to note that oxaliplatin potentiated radiation better than 5FU also in the V79 cells (data not shown). Thus, we feel confident that our results presented on the S1 cell line and evaluated by MTT are valid, in the radiation dose range up to approximately 4 Gy.
In conclusion, this study on the S1 colon cancer cell line confirms a synergistic cytotoxic effect between oxaliplatin and 5FU. Our results also suggest that oxaliplatin may be a better radiosensitizer than 5FU and that 5FU enhances radiation more effectively after long-term than short-term exposure. To confirm this observation, complementary studies on other cell lines and in vivo systems are needed. The best radiosensitizing effect was observed with oxaliplatin plus long-term 5FU exposure, supporting further clinical trials with oxaliplatin together with continuous 5FU treatment during radiotherapy. In the clinical setting it is quite inconvenient to administer 5FU intravenously during extended time periods. To replace intravenous 5FU with an oral fluoropyrimidine such as capecitabine, which easily can be administered during the whole radiotherapy period, seems like an attractive alternative. Several clinical studies are now investigating this avenue, especially in locally advanced rectal cancer.
Supported in part by grants from the Swedish Cancer Foundation (grant 4226-B01-03XAB) and Gunnar Nilsson′s Cancer Foundation. The authors also wish to thank Christina Boll for technical assistance and Pär-Ola Bendahl for statistical analyses.
References
- Parkin DM. Global cancer statistics in the year. Lancet Oncol 2000; 2: 533–43
- Ozer H, Diasio RB. Perspectives in the treatment of colorectal cancer. Semin Oncol 2004; 31: 14–8
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 2003; 3: 330–8
- Sobrero AF, Aschele C, Bertino JR. Fluorouracil in colorectal cancer–a tale of two drugs: implications for biochemical modulation. J Clin Oncol 1997; 15: 368–81
- Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 2001; 19: 2282–92
- Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, et al. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 1998; 352: 1407–12
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000; 355: 1041–7
- Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 2000; 18: 136–47
- de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938–47
- Sorbye H, Glimelius B, Berglund A, Fokstuen T, Tveit KM, Braendengen M, et al. Multicenter phase II study of Nordic fluorouracil and folinic acid bolus schedule combined with oxaliplatin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 2004; 22: 31–8
- Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level. (Review). Oncol Rep 2003; 10: 1663–82
- Carde P, Laval F. Effect of cis-dichlorodiammine platinum II and X rays on mammalian cell survival. Int J Radiat Oncol Biol Phys 1981; 7: 929–33
- Dewit L. Combined treatment of radiation and cisdiamminedichloroplatinum (II): a review of experimental and clinical data. Int J Radiat Oncol Biol Phys 1987; 13: 403–26
- Hess S, Blackstock W. Oxalipaltin: In vitro and In Vivo Evidence of its Radiation Sensitizing Activity-Pre-Clinical Observations Relevant to Ongoing Clinical Trials. 91st annual meeting of the American Association for Cancer research. Vol 2000; 41: 335
- Cividalli A, Ceciarelli F, Livdi E, Altavista P, Cruciani G, Marchetti P, et al. Radiosensitization by oxaliplatin in a mouse adenocarcinoma: influence of treatment schedule. Int J Radiat Oncol Biol Phys 2002; 52: 1092–8
- Freyer G, Bossard N, Romestaing P, Mornex F, Chapet O, Trillet-Lenoir V, et al. Addition of oxaliplatin to continuous fluorouracil, l-folinic acid, and concomitant radiotherapy in rectal cancer: the Lyon R 97-03 phase I trial. J Clin Oncol 2001; 19: 2433–8
- Johnsson, A, Kjellén, E. Early experience of long term radiotherapy combined with oxaliplatin, 5-FU and leucovorin in locally advanced rectal cancer. European Society for Medical Oncology annual meeting; 1992. p. 323.
- Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res 1999; 59: 8–13
- Stickel F, Jungert B, Brueckl V, Schirner I, Brueckl WM, Mannlein G, et al. Weekly high-dose 5-fluorouracil as 24-h infusion and folinic acid (AIO) plus irinotecan as second- and third-line treatment in patients with colorectal cancer pre-treated with AIO plus oxaliplatin. Anticancer Drugs 2003; 14: 745–9
- Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res 1987; 47: 943–6
- Kano Y, Suzuki K, Akutsu M, Suda K, Inoue Y, Yoshida M, et al. Effects of CPT-11 in combination with other anti-cancer agents in culture. Int J Cancer 1992; 50: 604–10
- Fischel JL, Etienne MC, Formento P, Milano G. Search for the optimal schedule for the oxaliplatin/5-fluorouracil association modulated or not by folinic acid: preclinical data. Clin Cancer Res 1998; 4: 2529–35
- Raymond E, Buquet-Fagot C, Djelloul S, Mester J, Cvitkovic E, Allain P, et al. Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast and ovarian cancers. Anticancer Drugs 1997; 8: 876–85
- Esaki T, Nakano S, Tatsumoto T, Kuroki-Migita M, Mitsugi K, Nakamura M, et al. Inhibition by 5-fluorouracil of cis-diamminedichloroplatinum(II)-induced DNA interstrand cross-link removal in a HST-1 human squamous carcinoma cell line. Cancer Res 1992; 52: 6501–6
- Balart J, Capella G, de los Inocentes RM, de Andres J, Ares C, Craven-Bartle J, et al. Treatment with 5-fluorouracil enhances radiosensitivity of the human pancreatic cancer cell line MiaPaCa-2. Pancreatology 2002; 2: 40–5
- Smith TA, Maisey NR, Titley JC, Jackson LE, Leach MO, Ronen SM. Treatment of SW620 cells with Tomudex and oxaliplatin induces changes in 2-deoxy-D-glucose incorporation associated with modifications in glucose transport. J Nucl Med 2000; 41: 1753–9
- Riccardi A, Ferlini C, Meco D, Mastrangelo R, Scambia G, Riccardi R. Antitumour activity of oxaliplatin in neuroblastoma cell lines. Eur J Cancer 1999; 35: 86–90
- Banasiak D, Barnetson AR, Odell RA, Mamegham H, Russell PJ. Comparison between the clonogenic, MTT, and SRB assays for determining radiosensitivity in a panel of human bladder cancer cell lines and a ureteral cell line. Radiat Oncol Investig 1999; 7: 77–85