Abstract
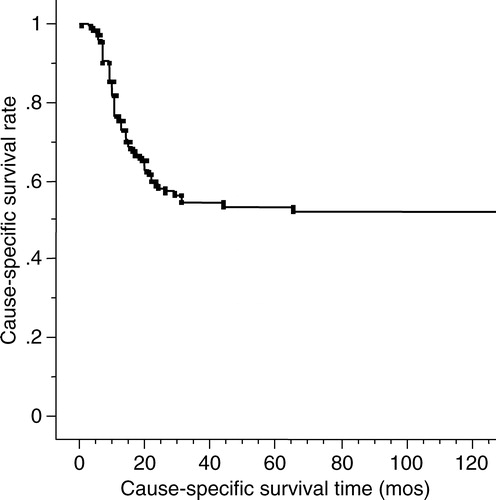
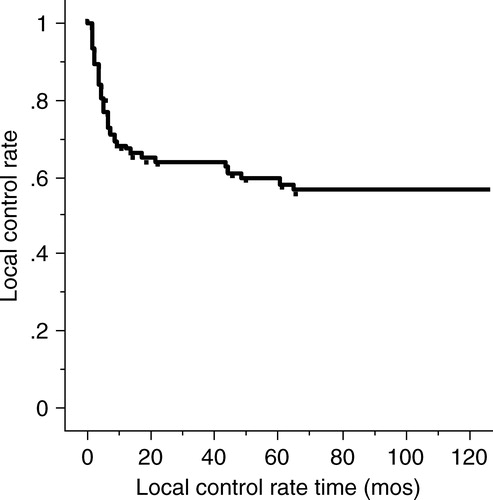
The purpose of this study is to describe the cause-specific survival rate, local control rate, salvage rate of neck metastasis, and post-treatment eating and speaking conditions for stage III mobile tongue squamous cell carcinomas and its subgroups. Between 1968 and 1999, 117 previously untreated patients with stage III mobile tongue carcinomas underwent mainly brachytherapy with external beam irradiation (EBRT) and neck dissection. A multivariate analysis was performed for the cause-specific survival rate on the various factors. The 1-, 3- and 5-year cause-specific survival rates for all patients were 76%, 54% and 54%, respectively. The 1-, 3- and 5-year primary control rates for all patients were 67.6%, 63.4% and 59.2%, respectively. There were statistically significant differences in cause-specific survival rates among stage III subgroups of T3N0, T1-2N1 and T3N1 (p = 0.0002). Our treatment method for patients with stage III mobile tongue squamous cell carcinoma was effective and acceptable.
The purpose of treatment for squamous cell carcinoma of mobile tongue is to control the tumor while preserving the structure and function in terms of speaking and eating without serious complications Citation[1–3]. An ideal treatment modality for advanced mobile tongue carcinoma has not yet been established and radiotherapy, surgery and chemotherapy have been used alone or in different combinations to achieve better treatment results Citation[1], Citation[3–15]. Interstitial brachytherapy and neck dissection have also been an option for advanced tongue carcinoma, allowing the disadvantages of surgery to be avoided Citation[5].
Only a few reports of different treatment modalities have examined the survival rates and the prognostic factors of stage III squamous cell carcinoma of the mobile tongue Citation[1], Citation[3–15]. Further study is therefore necessary to provide insight into the post-treatment condition following different treatment modalities of stage III mobile tongue carcinoma. The present study analyzed the cause-specific survival rate, local control rate, salvage rate of neck metastasis, and post-treatment eating and speaking conditions to identify an optimal treatment policy for each subgroup of stage III mobile tongue carcinoma.
Material and methods
From September 1968 to September 1999, 120 previously untreated patients with stage III mobile tongue squamous cell carcinoma visited the Department of Radiology of Tokyo Medical and Dental University Hospital. Three of them were excluded from this study because of dementia or being too old. This study included 117 previously untreated patients (78 men and 39 women) with stage III mobile tongue squamous cell carcinoma who were treated with interstitial brachytherapy with/without external beam irradiation (EBRT) combined with neck dissection. The ages of patients ranged from 25 to 92 years (median: 56 years). The histological diagnosis was squamous cell carcinoma in all patients.
The patients were re-staged retrospectively using the UICC (International Union Against Cancer 2002) TNM classification Citation[16]. Stage III mobile tongue cancer comprises T1N1M0, T2N1M0, T3N0M0 and T3N1M0. T1-tumor is defined as a primary tumor of 2 cm or less, T2 as more than 2 cm but not exceeding 4 cm, and T3 as more than 4 cm in greatest dimension. N0 is defined as no regional lymph node metastasis and N1 metastasis as a single ipsilateral lymph node, 3 cm or less in greatest dimension. M0 means no distant metastasis. In this study, the size of primary tumors was measured using calipers. Neck node metastases were evaluated primarily with palpation and confirmed with ultrasonography, computed tomography or magnetic resonance imaging. Chest radiograph was used for the evaluation of lung metastasis. As for the distribution of classifications in our study subjects (), the 117 stage III mobile tongue carcinoma patients comprised 68 T3N0M0, 1 T1N1M0, 28 T2N1M0, and 20 T3N1M0. The size of the primary tumor ranged from 2.0 cm to 6.0 cm (median: 4.3 cm) in the greatest dimension and a thickness from 0.1 cm to 5 cm (median: 1.8 cm). With regard to macroscopic appearance, 18 patients had superficial, 18 papillary or fungating (exophytic in the present report), and 81 ulcerative or infiltrating (invasive in the present report) tumors Citation[17].
Table I. Distribution of the 117 cases according to TNM classification (2002). Distribution of patients and tumor characteristics (117cases)
The primary tumors of 30 patients were treated with low dose rate interstitial brachytherapy alone and 87 patients were treated with a combination of brachytherapy with EBRT. The interstitial brachytherapy was performed under local anesthesia with an Au-198/Rn-222 seed in 21 patients (17.9%), a Cs-137/Ra-226 needle in 63 patients (53.8%), and an Ir-192 pin in 33 patients (28.2%). These different sources for brachytherapy were selected according to the size of primary tumors and patients’ conditions. Cs137/Ra-226 needle was used for patients with primary tumors over 1.5 cm in thickness and Ir-192 pin was used for those less than 1.5 cm. Complicated or handicapped patients were treated with Au-198/Rn-222 seed. The doses of interstitial brachytherapy for all patients ranged from 50 to 85 Gy (median: 70 Gy). The doses of interstitial brachytherapy when combined with EBRT ranged from 50 to 85 Gy (median: 67Gy). Pretreatment estimations of treatment time were made using Paterson-Parker's table and 70 Gy was set as the optimal dose for brachytherapy alone cases. The brachytherapy doses when combined with EBRT ranged from 50 to 70 Gy (median: 65Gy). Since 1976, the actual dose distribution has been obtained by computer dosimetry using rectangular X-ray films taken about 24 hours after implantation so as to deliver 70 Gy to the target volume. Therefore, treatment time was determined after calculating the dose distribution in 88 of all 117 patients. EBRT of 10 to 60 Gy (median: 30 Gy) was done before brachytherapy with single lateral portal in 74 patients and opposed lateral portals in 13 patients including the upper jugular region. The tumor dose of EBRT ranged from 12 to 55 Gy (median: 30 Gy) for those who had a single lateral portal. Brachytherapy performed two or three weeks following EBRT after the disappearance of mucositis and the target area was settled toward 5 mm outside the palpable mass. EBRT was routinely performed in the last half of the total period and was indicated for patients with a tumor thickness exceeding 2 cm and/or invasive primary lesions. Most patients in this period were treated with a single-plane implant with or without an extra-needle implant instead of a two-plane implant. EBRT was done for tumor bulk reduction as well as lowering the target volume. As a result, a two-plane implant became less frequent in the last half of the period (7 of 44 patients: 16%) compared to the high frequency (46 of 73 patients: 63%) in the first half.
Spacers made of translucent acrylic resin were introduced in 1987 and have been used since then. The thickness of the lingual part was designed to achieve approximately 10 mm with a minimum of 7 mm. The absorbed dose and dose rate at the lingual side surface of the lower gingiva prescribed by brachytherapy was assumed to be 50% of the tumor dose in the presence of a spacer Citation[18]. Eighty-one of the 117 patients were treated prior to introduction of the spacer and 36 patients were treated with a spacer.
Clinical neck lymph node metastasis was treated primarily by surgery, but EBRT was undertaken for patients who were contraindicated for, or refused surgery. No prophylactic neck dissection was performed in patients with T3N0M0, who were observed with “watch and see policy” for neck node metastasis. The follow-up period for all patients was 2 to 322 months (median: 35 months) from the date of the first visit until death or the last visit.
The cause-specific survival rate for each group was calculated according to the Kaplan-Meier method and was compared using the Mantel-Haenszel log-rank test. A multivariate analysis was performed on the following factors using the Cox proportional hazard model: gender, age, T classification, macroscopic appearance and thickness of primary tumor, N classification, radioactive sources and with or without EBRT. The χ2 test for independence was used for the analyses of the primary tumor recurrence and incidence of neck node metastasis with each macroscopic appearance and their results according to each treatment modality. All statistical analyses were performed with StatView software (SAS Institute, Cary NC). A p value of less than 0.05 was considered statistically significant.
Results
Fifty-four patients died of mobile tongue carcinoma, 29 patients died of other causes and 34 patients were alive at the time of writing. The 1-, 3- and 5-year cause-specific survival rates for all patients were 76%, 54% and 54%, respectively (). With regard to the sub-groups of stage III of the TNM classification, the 1-, 3- and 5-year cause-specific survival rates for 68 T3N0 patients were 90%, 67% and 67%, respectively. The 1-, 3- and 5-year cause-specific survivals for T1-2N1 and T3N1 patients were 67%, 37% and 33% and 40%, 35% and 35%, respectively (p = 0.0002) (). As for the macroscopic appearance of the primary tumor, the 1-, 3- and 5-year cause-specific survivals for the superficial type were 94%, 88% and 88%, respectively. The 1-, 3- and 5-year survival rates for the exophytic and invasive types were 83%, 66% and 60% and 69%, 45% and 45%, respectively (p = 0.004) (). Both univariate and multivariate analyses showed a significant difference in the different subgroups of stage III and primary tumor macroscopic appearance for the cause-specific survival rates (). Fifty-six patients who had a primary tumor over in 2 cm thickness showed no significant difference in the cause-specific survival rate compared with the patients with tumors less than 2 cm thick.
Figure 2. The cause-specific survival rates according to the T and N classifications. Subgroups of stage III show a statistical significance with close relationship to the presence of neck node metastasis.
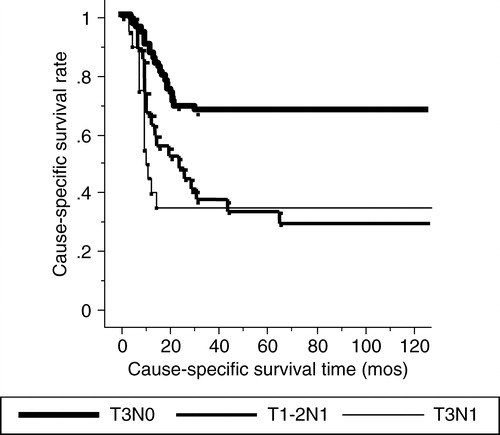
Figure 3. The cause-specific survival rates according to the macroscopic type. Invasive and exophytic types show poor prognosis compared to superficial type with statistical significance.
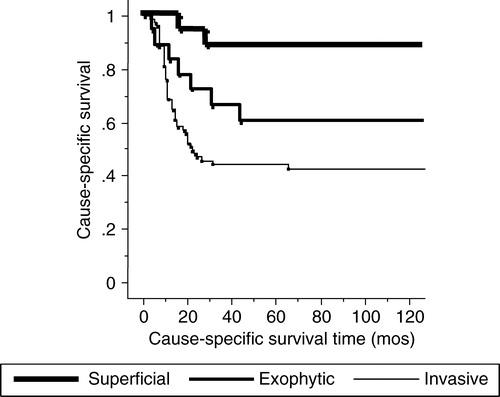
Table II. Prognostic factors in 117 patients for cause-specific survival
Of all 117 patients, 46 (39%) experienced primary tumor recurrences from 1 month to 65 months (median: 5 months) after the initial treatment. Three of 18 superficial type (17%), 8 of 18 exophytic type (44%), and 35 of 81 (43%) invasive type of tumors recurred. Primary tumor recurrence was significantly lower in the superficial type than in the exophytic and invasive types (p = 0.033). The primary tumor recurrence rate of T3-tumor was 39% (34 of 88 patients). According to the macroscopic appearance of T3-tumors, the recurrence rate of the superficial type was 21% (3 of 14 patients), that of the exophytic type 41% (7 of 17 patients), and that of the invasive type 42% (24 of 57 patients). There were no statistically significant differences in the control rate among these groups (p = 0.35). The 1-, 3- and 5-year primary control rates for all patients were 68%, 63% and 59%, respectively (). No significant statistical difference in local control rates was noted on univariate or multivariate analyses according to the T classification, N classification, macroscopic appearance, primary tumor thickness, or treatment source (). There was no difference in the primary control rates between 11 patients given 60 Gy by brachytherapy and 76 patients given over 60 Gy in the combined brachytherapy and EBRT (p = 0.42). EBRT brought about good primary control for stage III mobile tongue carcinoma on multivariate analysis (p = 0.010) (). The incidence of local atrophy and/or muscular fasciculation of the tongue were high in patients EBRT performed with the two plane implant (9 of 19 patients) rather than those with single plane implant (1 of 21 patients). Among those whose primary tumor could be followed over 2 years, local atrophy and/or muscular fasciculation occurred one to 7 years (median: 2 years and 3 months) following brachytherapy.
Salvation treatment for primary tumor recurrence was successful in 50% (13 of 26 patients) with surgery, 9.1% (1 of 11 patients) with radiation and 0% (0 of 8 patients) with observation (). Surgical salvage was statistically significant for primary tumor recurrence compared with radiation therapy and follow-up (p = 0.001).
Table III. Primary tumor and neck node metastasis controls according to macroscopic types
On admission, 49 patients (44.9%) had neck node metastasis and 28 (41.2%) of the T3N0 patients experienced neck node metastasis after primary tumor treatment. The overall incidence of neck node metastasis according to each primary macroscopic appearance was 39% (7 of 18 patients) for the superficial, 56% (10 of 18 patients) for the exophytic, 74% (60 of 81 patients) for the invasive type. These differences were statistically significant (p = 0.01). In terms of the criteria used for selection to treat patients with neck node metastasis, surgery was chosen for those indicated for neck dissection. EBRT was added to neck dissection in patients with extra-nodal invasion or more than three lymph node metastases. EBRT alone was performed in patients who refused or were contraindicated for surgery. The treatment of neck node metastasis was successful in 46% (24 of 54 patients) for surgery. Post-surgery EBRT of 25 – 50Gy (median: 40Gy) was given to 15 patients and was successful in 7 (47%). Neck dissection without post-surgery EBRT was done in 39 patients and was successful in 17 (44%). There was no significant difference between those with and without EBRT after neck dissection. Twenty-one patients with neck node metastasis underwent EBRT without neck dissection of 40 – 80 Gy (median: 55Gy) after primary tumor treatment with a success rate of 24% (5 of 21 patients). All 5 patients successfully treated with EBRT alone for neck node metastasis were irradiated over 50 Gy. Patients who could not be treated surgically or with radiation had an effective treatment rate of 0% (0 of 2 patients) (.). Surgery was statistically dominant for neck node metastasis compared with EBRT alone and follow-up (p = 0.043).
Local radiation ulcer or fibrosis necessitating surgery occurred in 2 of 11 patients (18.2%) who were treated without computer dosimetry and in whom the primary lesions could be followed over 2 years. There were no such overdose complications after the introduction of computer dosimetry. Mandibular osteoradionecrosis necessitating surgery occurred in 8 of 34 patients (23.5%) treated without a spacer. Another 4 patients with mandibular osteoradionecrosis without using a spacer were managed conservatively. No osteoradionecrosis was seen after the introduction of a spacer. There were only 8 local ulcers of brief duration after mucositis among 25 locally cured patients treated with a spacer. Two patients showed grade 2 salivary gland late effects of permanent xerostomia, who were given 40 and 50 Gy of EBRT by parallel opposed field technique. There were no patients complaining salivary gland function treated with a single lateral field EBRT technique. Post-treatment eating and speaking conditions was evaluated on the basis of patients’ signs and symptoms at outpatient consultations. In almost all patients, the oral conditions of speaking and eating were not different from those of healthy persons.
Sixteen secondary or tertiary cancers were found in 14 patients (12.0%). One of these cancers was synchronous and 15 were metachronous. The origins were the esophagus in 6 patients, the lung in 5 patients, stomach in 2 patients, sigmoid colon in 1 patient, skin in 1 patient, and ovary in 1 patient. As has been previously noted, a high incidence of multiple primary cancers was evident in the upper aero-digestive tract Citation[19].
Discussion
It is obvious that the stage of tumor plays a significant role in the selection of treatment modalities for oral tongue carcinoma Citation[7]. For patients with early mobile tongue carcinoma, interstitial brachytherapy or surgery Citation[20], Citation[21] has been used with good survival and local control rates while preserving oral structure and function Citation[5]. For advanced cases, the main treatment modalities for the primary tumor have been surgical, however, different combinations of surgery, radiotherapy and chemotherapy have been tried to obtain optimal post-treatment conditions Citation[1], Citation[3–15]. EBRT alone was reported to be rarely successful and not sufficient to eradicate larger primary tumors Citation[13], Citation[14]. Interstitial brachytherapy alone or in combination with EBRT has been reported to achieve better results concerning local control Citation[1], Citation[5], Citation[8], Citation[15], Citation[19], Citation[21]. In addition, a number of operative specimens obtained after combined interstitial brachytherapy and EBRT were histologically negative or contained relatively small numbers of tumor cells Citation[14]. Therefore, interstitial brachytherapy combined with EBRT could be selected as one of the less invasive as well as less complicated curative treatment modalities for advanced tongue carcinoma, especially in elderly patients or those with systemic diseases, and those who refuse surgery, while hoping to preserve better oral function.
Various survival rates with advanced mobile tongue carcinoma treated either with radiotherapy, surgery, or a combination of these has been reported Citation[2], Citation[5], Citation[12], Citation[13], Citation[15]. As for the survival rates of those treated mainly with surgery, Cummings et al. Citation[8] reported a cure rate of 31% in patients treated with initial surgery at stage III and IV according to AJCC 1976 staging. Franceschi et al. Citation[6] reported a 5-year determinate survival rate of 49% in stage III and IV patients according to the 1988 AJCC classification and Pukander J et al. Citation[7] reported a 5-year overall survival rate of 27.7% in stage III patients according to the same classification. Some institutions have reported results of T3-tumors treated with brachytherapy alone or a combination with EBRT and interstitial brachytherapy Citation[2], Citation[4], Citation[5], Citation[15]. The local control rate for T3-tumor was reported to be about 32 – 71% in these studies. Hareyama et al. Citation[2] reported the local-recurrence-free rate for interstitial brachytherapy with/without EBRT to be 71% for T3 classification (UICC 1987). Kakimoto et al. reported a 3-year control rate of 68% following low- and high dose-rate brachytherapy Citation[5]. In our study, the primary lesion control rate of T3-tumors was 61% and the 1-, 3- and 5-year cause-specific survival rates for all stage III patients were 76%, 54% and 54% respectively. Although the patients’ backgrounds and carcinoma stages were different from those in previous reports, our data on interstitial brachytherapy and EBRT for T3-tumors were comparable to the surgical data or other brachytherapy data. In our institution, sufficient radiation doses with a low complication rate were obtained after the introduction of the computer dosimetry and the spacer – a mandibular protector that keeps the tongue and mandible apart and the policy of single plane radioactive source implantation. Our spacer was assumed to reduce about 50% of the absorbed dose at the lingual side of the lower gingiva to that in the absence of a spacer Citation[18]. Post-brachytherapy oral conditions of patients have become acceptable since the introduction of these two modalities. The EBRT doses with interstitial brachytherapy being maintained below the level of salivary gland complications have also resulted in minimal salivary dysfunction Citation[22].
In an early report published by Paterson Citation[17], the macroscopic appearance of tongue carcinoma was categorized into four types based on the point of the extent and direction of invasion; papillary or fungating, superficial, ulcerative and infiltrating. Superficial lesions spread superficially without much palpable infiltration, commonly as the end result of leukoplakia. The papillary or fungating type and the ulcerative and infiltrating types were referred to in the present report as the “exophytic type” and “invasive type”, respectively. At the base of the exophytic type, the tongue carcinoma slightly invaded adjacent normal tissue. The invasive type was the most common and typical form of advanced tongue carcinoma with a tendency to invade deeply into the tongue. In the present study, 69% of all cases showed an invasive growth pattern and our 1-, 3- and 5-year cause-specific survival rates were statistically significant among the macroscopic appearances types (superficial: 94%, 88% and 88%; exophytic: 83%, 66% and 60%; invasive: 69%, 45% and 45%). The invasive and exophytic types had a significant primary recurrence rate compared with the superficial type. The invasive type showed a high incidence of neck metastases and the lowest cause-specific survival rate, with statistical significance. The invasive type had the worst macroscopic appearance in regard to the cause-specific survival and neck node metastasis, followed by the exophytic and superficial types. In patients with early stage tongue carcinoma, the macroscopic appearance of the primary tumor is reported as the dominant factor for predicting lymph node metastasis Citation[20]. The small difference between macroscopic types may suggest that interstitial brachytherapy with EBRT is a reasonable treatment for exophytic and invasive tumors. Combining unilateral 30 – 40 Gy of EBRT and 60 Gy of single plane interstitial brachytherapy appears to be an optimal treatment modality for T3 exophytic and invasive tumors. Compared with the invasive and exophytic types, the superficial type seemed to be less aggressive and was assumed to be curable by interstitial brachytherapy alone.
Our 1-, 3- and 5-year cause specific survival rates were definitely different among the subgroups of stage III (T3N0: 90%, 67% and 67%; T1-2N1: 67%, 37% and 33%; T3N1: 40%, 35% and 35%). Survival data were closely related to the presence of neck node metastasis. As for the management of neck node metastasis, surgery has been considered as the best treatment of choice. Some authors advocated surgery plus irradiation for more advanced tumors with unilateral neck node metastases, because of the better results achieved than with irradiation alone Citation[3]. In general, there is a tendency that the more advanced the primary tumor, the higher the incidence of neck node metastasis Citation[24]. Treatment failure of neck lymph node metastasis was the most significant problem for the survival rate Citation[7], Citation[20], Citation[25]. In the initial stage, lymph nodes play a role as a filter and barrier function in echelon, however, as a lymph node is replaced by metastatic tumor and after the extra-capsular spread, the cancerous node itself may act as a focus for distant metastasis Citation[20], Citation[24]. As for the survival rate of those positive for neck metastases, Vermund et al. reported a 5-year cause-specific survival of 28% for patients with TxN1 treated with irradiation and surgery Citation[12]. Hareyama et al. Citation[2] reported a 5-year actuarial survival rate for patients with T2 + 3N1 + 2 of 51.6%. Several authors Citation[1], Citation[13], Citation[20] reported that neck node metastasis was one of the significant factors for survival on multivariate analysis. Our results of cause-specific survival were consistent with these reports and showed that the differences in survival rate among the different subgroups of stage III resulted from the neck node condition. Elective nodal irradiation with a median dose of 30 Gy was inadequate to control nodal metastasis. Thus, over 40 Gy is necessary to reduce neck metastasis with prophylactic irradiation for clinically negative regional lymph nodes Citation[21], however higher doses were associated with complications for long-term survivors.
Radical neck dissection is reported the most successful treatment for neck lymph node metastases Citation[8]. Vermund et al. Citation[3] described surgery combined with EBRT as better than moderate doses of radiation alone. With cases of early mobile tongue carcinoma, treatments of the clinically negative neck including cervical lymph-node dissection or radiotherapy were advocated, since some cases had tumors that behaved like stage III disease Citation[8]. In the present study, the surgical control rate of neck node metastasis was 46.3%, which was statistically dominant compared with that of 23.8% following radiotherapy or 0% with observation. There was no significant difference between those treated with and without EBRT after neck dissection. Treatment failure of neck lymph node metastasis was the most significant problem affecting the survival rate Citation[7], even among patients whose primary tumor had been cured.
In conclusion, the treatment method of interstitial brachytherapy and neck dissection for patients with stage III mobile tongue squamous cell carcinoma was effective and acceptable, especially for primary tumors, with the advantage of preserving oral condition. To the best of our knowledge, there have been no reports on prognostic factors confined to subgroups of stage III mobile tongue carcinoma. We investigated the relationships among various possible prognostic factors and found N stage to be the dominant factor for predicting cause-specific survival. The macroscopic appearance of the primary tumor was the second most significant factor according to our univariate and multivariate analyses. Computer dosimetry, spacer use, and single plane implant of radioactive source were essential to achieve better post-treatment eating and speaking conditions.
References
- Nyman J, Mercke C, Lindstrom J. Prognostic factors for local control and survival of cancer of the oral tongue. A retrospective analysis of 230 cases in western Sweden. Acta Oncol 1993; 32: 667–73
- Hareyama M, Nishio M, Saito A, Kagami Y, Asano K, Oouchi A, et al. Results of cesium needle interstitial implantation for carcinoma of the oral tongue. Int J Radiat Oncol Biol Phys 1993; 25: 29–34
- Vermund H, Brennhovd IO, Kaalhus O, Poppe E. Squamous-cell carcinoma of the tongue: preoperative interstitial radium and external irradiation. Part I: Local and regional control. Radiology 1984; 151: 499–503
- Horiuchi J, Okuyama T, Shibuya H, Takeda M. Results of brachytherapy for cancer of the tongue with special emphasis on local prognosis. Int J Radiat Oncol Biol Phys 1982; 8: 829–35
- Kakimoto N, Inoue T, Inoue T, Murakami S, Furukawa S, Yoshida K, et al. Results of low- and high-dose-rate interstitial brachytherapy for T3 mobile tongue cancer. Radiother Oncol 2003; 68: 123–8
- Franceschi D, Gupta R, Spiro RH, Shah JP. Improved survival in the treatment of squamous carcinoma of the oral tongue. Am J Surg 1993; 166: 360–5
- Pukander J, Karhuketo T, Penttila M, Pertovaara H, Karma P. Radical surgery for lingual cancer. Clin Otolaryngol 1990; 15: 229–34
- Leipzig B, Cummings CW, Chung CT, Johnson JT, Sagerman RH. Carcinoma of the anterior tongue. Ann Otol Rhinol Laryngol 1982; 91: 94–7
- Yarington CT, Jr. A protocol for combined treatment of cancer of the oral cavity and tongue. Laryngoscope 1980; 90: 2004–8
- White D, Byers RM. What is the preferred initial method of treatment for squamous carcinoma of the tongue?. Am J Surg 1980; 140: 553–5
- Haddadin KJ, Soutar DS, Webster MH, Robertson AG, Oliver RJ, MacDonald DG. Natural history and patterns of recurrence of tongue tumours. Br J Plast Surg 2000; 53: 279–85
- Vermund H, Brennhovd IO, Kaalhus O, Poppe E. Squamous-cell carcinoma of the tongue: preoperative interstitial radium and external irradiation. Part II: Survival. Radiology 1984; 151: 505–8
- El-Husseiny G, Kandil A, Jamshed A, Khafaga Y, Saleem M, Allam A, et al. Squamous cell carcinoma of the oral tongue: an analysis of prognostic factors. Br J Oral Maxillofac Surg 2000; 38: 193–9
- Vermund H, Jacobsen AB, Kaalhus O, Levernes S, Melsom H, Tausjo J, et al. Changing aspects in the treatment of squamous cell carcinoma of the oral tongue. Acta Oncol. 1987; 26: 163–72
- Pernot M, Malissard L, Hoffstetter S, Luporsi E, Peiffert D, Aletti P, et al. The study of tumoral, radiobiological, and general health factors that influence results and complications in a series of 448 oral tongue carcinomas treated exclusively by irradiation. Int J Radiat Oncol Biol Phys 1994; 29: 673–9
- Sobin LH, Wittekind Ch, editors. UICC TNM Classification of Malignant Tumors. 6th ed. New York: John Wiley & Sons, Inc.; 2002 .
- Paterson R. The treatment of malignant disease by radiotherapy. Edward Arnold Publishers, London 1963
- Miura M, Takeda M, Sasaki T, Inoue T, Nakayama T, Fukuda H, et al. Factors affecting mandibular complications in low dose rate brachytherapy for oral tongue carcinoma with special reference to spacer. Int J Radiat Oncol Biol Phys 1998; 41: 763–70
- Yuen AP, Lam KY, Chan AC, Wei WI, Lam LK, Ho WK, et al. Clinicopathological analysis of elective neck dissection for N0 neck of early oral tongue carcinoma. Am J Surg 1999; 177: 90–2
- Nakagawa T, Shibuya H, Yoshimura R, Miura M, Okada N, Kishimoto S, et al. Neck node metastasis after successful brachytherapy for early stage tongue carcinoma. Radiother Oncol 2003; 68: 129–35
- Shibuya H, Hoshina M, Takeda M, Matsumoto S, Suzuki S, Okada N. Brachytherapy for stage I & II oral tongue cancer: an analysis of past cases focusing on control and complications. Int J Radiat Oncol Biol Phys 1993; 26: 51–8
- Murata Y, Zhang L, Ishida R, Aung W, Taura S, Hossain M, et al. Maintained salivary function after brachytherapy in patients with head and neck carcinomas--evaluation using quantitative salivary gland scintigraphy. Acta Oncol 2002; 41: 684–8
- Nithya C, Pandey M, Naik B, Ahamed IM. Patterns of cervical metastasis from carcinoma of the oral tongue. World J Surg Oncol 2003; 1: 10
- Snow GB, Patel P, Leemans CR, Tiwari R. Management of cervical lymph nodes in patients with head and neck cancer. Eur Arch Otorhinolaryngol 1992; 249: 187–94
- Takagi M, Kayano T, Yamamoto H, Shibuya H, Hoshina M, Shioda S, et al. Causes of oral tongue cancer treatment failures. Analysis of autopsy cases. Cancer 1992; 69: 1081–7