Abstract
Skeletal metastases are a significant problem in prostate cancer (PC). The patients are also exposed to treatment-related skeletal changes. This cross-sectional study evaluated a marker of bone resorption, TRACP 5b in relation to the standard analyte total alkaline phosphatase (tALP) as a marker of skeletal changes. Serum levels of TRACP 5b, tALP and PSA were measured in 130 prostate cancer patients. Comparison was made between patients with (BM+, n = 25) and without (BM−, n = 105) skeletal metastases, and between those treated with (n = 64) or without (n = 66) androgen deprivation (AD). Sensitivities and specificities were calculated for each marker and diagnostic accuracy was evaluated by ROC curve analysis. ROC curves indicated the superior accuracy of tALP, whereas TRACP 5b and PSA were comparable. With tALP the best combination of sensitivity (96%) and specificity of (91%) was reached at a cut-off point 224 U/L, the corresponding values were for TRACP 5b sensitivity (76%), specificity (89%) with a cut-off point 4.89 U/L, and for PSA sensitivity (65%), specificity (81%) at 23 ng/L for skeletal metastases. Patients treated with AD showed with increasing duration an increase in TRACP 5b values. TRACP 5b was less specific than tALP as a marker of skeletal metastases. TRACP 5b may have a role in the diagnostics of skeletal changes in PC with a focus on treatment-related skeletal changes.
Prostate cancer (PC) is the most common cancer among Finnish men and the second common cause of cancer-related death Citation[1]. Skeletal metastases occur in the majority of patients with advanced PC Citation[2] and these can involve skeletal events and suffering to the patients and significant resource requirements and costs to the care providers. Skeletal metastases in PC are predominantly sclerotic and pathological fractures occur more commonly in PC than in other cancers metastasizing to bone Citation[3]. It is therefore important to evaluate markers predictive of skeletal changes among these patients. Many patients with PC are elderly, the mean age being in Finland 70 years at diagnosis, and will therefore often also evince other skeletal changes, e.g. due to degeneration and osteoporosis, which increase with aging. Treatment by surgical castration or androgen-deprivative drugs (AD) reduces bone mineral density and can lead to osteoporosis with consequent skeletal events Citation[4]. With the increasing use of AD also as neo + adjuvant treatment, significant therapy-related skeletal changes have been observed even in the absence of skeletal metastases Citation[4], Citation[5]. The evaluation of skeletal changes in PC patients is thus challenging and specific diagnostic markers need to be evaluated for their potential to improve their diagnostics and management.
The interaction between cancer and bone is largely mediated by the osteoclasts, irrespective of the underlying tumour type Citation[6]. Serum tartrate-resistant acid phosphatase 5b (TRACP 5b) is recognized as a marker of bone resorption, and it is synthesized and secreted into the circulation by osteoclasts in the course of bone resorption. Based on recent knowledge, all circulating TRACP 5b is produced by osteoclasts Citation[7], which makes it an excellent marker of bone resorption Citation[8]. Being measured from the serum, TRACP 5b is free from the potential errors associated with the markers quantitated in the urine. Additionally, TRACP 5b shows very low diurnal variation and is not affected by fasting or renal or hepatic failure Citation[9], Citation[10].
The aim of this cross-sectional study was to evaluate the potential value of serum TRACP 5b in clinical practice by comparing it to the routinely used tALP and PSA as a marker of skeletal metastases in PC.
Patients and methods
Serum samples were collected from 130 patients with a histologically confirmed diagnosis of PC attending the Department of Oncology, Turku University Hospital in the period of January 2000 to January 2003. All patients provided informed consent. The study was approved by the joint ethical committee of Turku University Hospital and the University of Turku.
Clinical data were collected from the patients and their files, including information on disease status, treatment of cancer and history of other diseases and medications, which could affect bone metabolism. The presence and type of skeletal metastases and presence of degenerative or osteoporotic skeletal changes was verified by expert radiologists reviewing X-rays films (MV). Twenty-five patients had skeletal metastases (BM + ), whereas 105 patients showed no signs of these (BM − ).
Non-fasting samples were collected before early afternoon and stored at −70°C. Total ALP was determined using a standard laboratory method (Roche Diagnostics GmbH Mannheim, Germany, with Hitachi 917 Hitachi Ltd, Tokyo, Japan) and PSA was determined using the TR-IFMA method (AutoDELFIA Wallac Finland OY, Turku, Finland). TRACP 5b activity was measured using an immunoassay as previously described by Halleen and associates Citation[11]. Monoclonal antibody O1A (200 ng/well) was incubated for 1 h in anti-mouse IgG-coated micro titer wells (Wallac Perkin Elmer, Turku, Finland). After washing, duplicate serum samples were incubated in the wells for 1 hour. Bound TRACP 5b activity was determined by incubating the wells for 1 hour in 0.1 M sodium acetate buffer, pH 6.1, containing 4-nitrophenylphosphate (4-NPP) as substrate. The reactions were stopped by adding sodium hydroxide to the wells, and A405 was determined using Victor II equipment (Wallac Perkin Elmer, Turku, Finland).
The samples were analyzed on two occasions meaning they were analysed in two runs. Assay reproducibility was determined by the following values: intra-assay CV 6.4%, inter-assay CV 7.6%. The CV's were measured using samples with three levels of activity: 1 U/L, 5 U/L and 10 U/L. Limit of detection is 0.06 U/L, linear range at least up to 20 U/L.
Statistical analyses
Groupwise comparison of the serum markers TRACP5b, tALP and PSA was made using the nonparametric Wilcoxon test. Spearman rank correlation was used to analyze associations between the serum markers, since the distributions of the markers were skewed to the right. The sensitivity and specificity of the markers using different cut-off points were calculated and ROC curves were constructed to evaluate and compare the markers as diagnostic tests to discriminate between BM+ and BM− groups. The best cut-off point was set at the point nearest to the corner in the ROC figure, where both sensitivity and specificity is 100%. The 95% confidence intervals were calculated for these sensitivity and specificity estimates with the standard normal distribution formula or with the exact binominal formula (if n < 100). The area under the ROC curve with a 95% confidence interval was calculated for each marker. Cohen kappa coefficients were calculated to assess the agreement between markers when the best cut-off points were applied. The kappa values were interpreted using the following guidelines: kappa value < 0.20 indicated poor agreement, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 good and 0.81–1.00 very good agreement. Analysis of variance (2-way ANOVA) was used to study the effect of hormone treatment and skeletal metastases on the logarithmically transformed TRACP 5b, tALP and PSA. Pearson correlation coefficients were calculated to test the linear relationship between the (ln) duration of hormone treatment and (ln) serum markers. Linear regression analysis was used to describe an observed significant relationship. The analyses were performed using SPSS (Version 12.0, SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics are summarized in . Two patient populations were defined, one with and the other without documented skeletal metastases. The mean age of all patients was 69 years (range 48–88). The presence of degenerative bone changes and osteoporosis was not significantly different between patients with or without skeletal metastases (p = 0.530).
Table I. Characteristics of prostate cancer patients with (BM + ) and without (BM − ) skeletal metastases.
Review of the X-ray films indicated that the majority of skeletal metastases were sclerotic (22/25, 88%), and only 3 of 25 patients (12%) had lytic or lytic/sclerotic metastases. Forty-three patients without skeletal metastases (41%) were not treated and 21 patients with skeletal metastases (84%) were treated with AD therapy.
The levels of tALP, TRACP 5b and PSA in the BM+ and BM− groups, respectively, are shown in . In general, the levels were higher among patients with (BM + ) skeletal metastases than among those without (p < 0.001). TRACP 5b values in lytic metastases were not specifically high (remaining < 6 U/L) compared to the sclerotic (data not shown).
In the BM− group the geometric means were 3.3 and 3.3 for TRACP 5b, 149.9 and 183.7 for tALP, and 0.5 and 15.0 for PSA in patients with and without degenerative changes. In the BM+ group the corresponding figures were 5.9 and 4.9 for TRACP 5b, 721.6 and 604.4 for tALP, and 16.9 and 70.1 for PSA. Two-sided analysis of variance using the logarithmically transformed values yielded a non-significant effect for degenerative changes (p = 0.425 for TRACP5b, p = 0.957 for tALP and p = 0.242 for PSA).
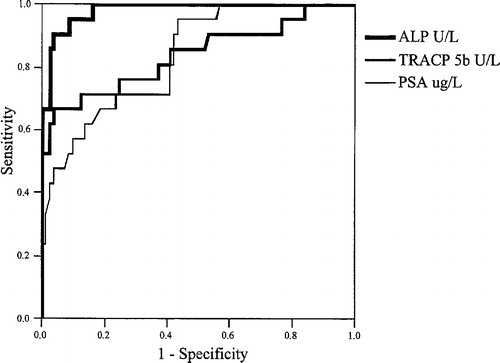
Analyses were made to characterize the usefulness of the markers in differentiating between PC patients with and without bone metastases. The ROC curves for tALP, TRACP5b and PSA in the detection of skeletal metastases in PC patients are presented in . The superior accuracy of tALP was seen, while the curves for TRACP5b and PSA did cross.
The areas under the ROC curves for the markers in discriminating between BM+ and BM− are given in . When tALP showed superior accuracy (area = 0.98) in comparison with TRAPC5b (area = 0.84) and PSA (area = 0.84), no significant difference was observed between the latter two.
Table II. Area under ROC curve for tALP, TRACP and PSA.
shows a comparison of the clinical specificity and sensitivity of tALP, TRACP5b and PSA at the cut-off points giving the best sensitivity and specificity combination. With tALP the best combination of sensitivity (96%) and specificity (91%) was reached at a cut-off point of 224 U/L. TRACP5b had the best combination of sensitivity (76%) and specificity (89%) with a cut-off 4.89 ug/L, and PSA had the best combination of sensitivity (65%) and specificity (81%) at 23 ug/L in discriminating between patients with and without metastases. Using the above cut-off points the agreement was good between TRACP5b and tALP (agreement = 85%, kappa = 0.61), fair between TRACP5b and PSA (agreement = 76%, kappa = 0.36) and moderate between tALP and PSA (agreement = 82%, kappa = 0.58).
Table III. Determination of clinical specificity and sensitivity of tALP, TRACP 5b and PSA for bone metastases of prostate cancer. The 95% confidence intervals are in parenthesis.
TRACP5b was significantly associated with tALP in both groups (BM+ r = 0.56, p = 0.006, BM− r = 0.32, p = 0.004) groups. The association between TRACP5b and PSA was statistically significant only in the BM+ group (r = 0.48, p = 0.021, in the BM− group r = − 0.03, p = 0.736), while between tALP and PSA an association was seen in both groups (in BM+ r = 0.28, p = 0.012 and in BM− r = 0.43, p = 0.051).
The effect of androgen deprivation (AD) on the markers was specifically noted. In the BM− group the geometric means were 3.3 and 3.1 for TRACP5b, 158.5 and 158.4 for tALP, and 0.6 and 1.9 for PSA in patients with and without hormone treatment. In the BM+ group the corresponding figures were 6.9 and 5.2 for TRACP5b, 751.3 and 455.4 for tALP, and 72.4 and 12.8 for PSA. Two-sided analysis of variance using the logarithmically transformed values of the markers yielded a non-significant effect for androgen deprivation (p = 0.330 for TRACP5b, p = 0.499 for tALP and p = 0.863 for PSA). The interactions were significant or almost significant for PSA (p = 0.044) and for tALP (p = 0.087), indicating that the effect of androgen deprivation may be different in patients with skeletal metastases compared to those without. Using the logarithmically transformed values, the duration of androgen deprivation was positively correlated with TRACP5b (r = 0.246, p = 0.050) and with tALP (r = 0.253, p = 0.076). The estimated regression line for TRACP5b was: ln (TRACP5b) = 0.089 * ln (Duration of AD) (months) + 1.23.
Six patients in the BM+ group had been treated with bisphosphonates. Their median TRACP 5b value was 8.4 U/L, which was slightly, but not significantly elevated compared to the median 6.3 U/L in nineteen BM+ patients not treated with bisphosphonates (p = 0.514). The other markers were likewise not significantly altered by bisphosphonates.
Discussion
Prostate cancer is the most common cancer among males and the second cause of cancer-related death among men in most Western countries. Advancing prostate cancer negatively impacts normal bone physiology, both by the destructive effect of metastases and by the osteoporotic effects of androgen deprivation therapy Citation[12]. To assess the clinical role of TRACP5b in the diagnostics of skeletal changes in prostate cancer, we here report that TRACP5b as a marker of skeletal changes in prostate cancer proved less specific than tALP in defining skeletal metastases. However, TRACP5b appeared to be influenced by androgen deprivation therapy, with a potential to predict treatment-related skeletal changes.
TRACP 5b has been measured successfully from human serum samples that have been stored at least up to 10 years at −70C. However, when serum samples are stored at −20C, TRACP 5b is stable only for 1–2 months. Here the samples were stored at −70C, which means that there are no stability problems. The marker, as all bone markers, is age- and sex-dependent. Values are very high in children during growth period, decrease to normal range in young adults and increase again during aging due to age-related osteoporosis. This is typical to all bone markers. Normal values (mean + SD) are presented in . The values for patients with skeletal metastases were clearly higher than those of healthy men. There was also tendency to higher values when patients were treated with androgen deprivation, which indicates that the marker is sensitive to decreased bone mineral density associated with this treatment Citation[4].
Table IV. Reference range of serum TRACP 5b activity in healthy adults. Upper normal limits (mean + 2 SD of young adults): Women: 4.82 U/L, Men: 5.34 U/L.
Patients with skeletal metastases and hormone refractory prostate cancer invariably develop bone pain, fractures, anaemia and other skeleton-related complications, and these patients have a survival of less than one year Citation[13]. The skeletal scintigram is routinely used in PC patients to monitor skeletal metastases, but its detection value has been shown to be low in early lesions and with low PSA levels Citation[14].
Compared to scintigram and radiological imaging, serum markers are easy and cheap in clinical practice. A review of ten different markers was recently published, suggesting that TRAP5b could be the preferred resorption marker for prostate cancer patients Citation[8], but its value as against tALP, which is used in clinical routine, needed to be assessed. The current results show that tALP can maintain its role as the routine serum marker for skeletal metastases in prostate cancer. This may be due to the predominantly sclerotic character of the skeletal metastases, as confirmed in the radiological re-evaluation.
The increasingly common use of androgen deprivation in the therapeutic management of even non-metastatic prostate cancer also affects the skeletal health of patients. As a result of androgen deprivation an increasing incidence of fracture risk, increasing with duration of therapy has been observed, especially when therapy effects are extended over several years Citation[15]. With the more common use of androgen deprivation as adjuvant treatment, if extended over several years, impairment of skeletal health can be expected in a growing number of prostate cancer patients. Osteoporosis and the resultant increase in fracture incidence occur in prostate cancer patients treated with androgen deprivation therapy Citation[12], Citation[16]. The development of pathological fractures is associated with impaired survival, even if the fractures are on the basis of osteoporosis without metastatic spread Citation[4], Citation[17]. Nonmetastatic patients receiving antiandrogen therapy have been reported to show only moderately increased serum tALP Citation[18], Citation[19]. A clinical tool easier than bone density measurements or radiological investigations would be welcome to survey PC patients for skeletal changes, as prostate cancer patients are increasingly exposed to long-lasting androgen deprivation with detrimental effects on bone health Citation[4], Citation[17]. TRAP5b levels increased with the increase in duration of AD and therefore its role as predictor of therapy-induced skeletal changes needs to be further studied.
Skeletal metastases can be difficult to differentiate from degenerative skeletal changes in elderly patients. In the present study TRACP5b appeared uninfluenced by skeletal degeneration and therefore proved to be a specific marker of skeletal changes in prostate cancer patients with radiological skeletal metastases. The advantage of laboratory tests compared to imaging technology is that they are non-invasive, cost-effective and easy to perform. However, the present findings and comparison with tALP, the usual marker in skeletal changes in prostate cancer, indicates that as a marker of metastatic skeletal changes TRACP5b is less specific, since it was influenced by androgen deprivation therapy, levels apparently increasing with increasing months of therapy. With the increasing use of androgen deprivation therapy also in an adjuvant setting, a marker for early skeletal changes is needed to predict the risk of skeletal events. There is a great opportunity to improve skeletal health among men receiving androgen deprivation treatment Citation[20] e.g. by using bisphosphonates Citation[21]. The present findings suggest that TRACP5b should be further studied with an eye to improving the clinical armament as a valuable marker to follow the skeletal health of PC patients. Prevention of the deleterious skeletal effects should improve the patient's quality of life and may affect patient survival Citation[12]. Intervention studies are required for the prevention of skeletal events with early detection and therapeutic intervention.
Conclusions
In conclusion due to the osteosclerotic nature of skeletal metastases in prostate cancer the widely used serum marker tALP has greater sensitivity than TRACP5b, a specific marker of bone destruction. As PSA is a marker of PC rather than skeletal metastases, its sensitivity is lower. The role of TRACP5b in predicting therapy-associated skeletal changes in prostate cancer needs to be further characterized.
References
- Cancer Incidence in Finland. Finnish Cancer Registry, Helsinki2000.
- Budendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1589 patients. Hum Pathol 2000; 31: 578–83
- Coleman RE. Skeletal complications of malignancy. Cancer 1997; 80: 1588–94
- Oefelein MG, Ricchuiti V, Conrad W, Seffel A, Bodner D, Goldman R, et al. Skeletal fractures associated with androgen suppression induced osteoporosis: the clinical incidence and risk factors for patient with prostate cancer. J Urol 2001; 166: 1724–8
- Salminen EK, Portin R, Korpela J, Helenius H, Parvinen L-M, Backman H, et al. Androgen deprivation and cognition in prostate cancer. Br J Cancer 2003; 89: 971–6
- Rodan GA. The development and function of the skeleton and bonemetastases. Cancer 2003; 97: 726–32
- Janckila AJ, Parthasarathy RN, Parthasarathy LK, Seelan RS, Yam LT. Stable expression of human tartrate-resistant acid phosphatase isoforms by CHO cells. Clin Chim Acta 2002; 326: 113–22
- Jung K, Lein M, Stephan C, Von Hösslin K, Semjonow A, Sinha P, et al. Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. Int J Cancer 2004; 111: 783–91
- Halleen JM, Alatalo SL, Janckila AJ, Woitge HW, Seibel MJ, Väänänen HK. Serum tartrate-resistant acid phosphatase 5b is a specific and sensitive marker of bone resorption. Clin Chem 2001; 47: 597–600
- Hannon RA, Clowes JA, Eagleton AC, Al Hadari A, Eastell R, Blumsohn A. Clinical performance of immunoreactive tartrate-resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone 2004; 34: 187–94
- Halleen JM, Alatalo SL, Suominen H, Cheng S, Janckila AJ, Vaatanen HK. Tartrate resistant acid phosphatase 5B: a novel serum marker of bone resorption. J Bone Miner Res 2000; 15: 1337–45
- Eastham JA. Bishosphanates and prostate cancer: Maintaining bone integrity and quality of life. Am J Urol Review 2004; 2(Suppl 2)5–8
- Tu SM, Millikan RE, Mengistu B, Delpassand SE, Amato RJ, Pagliaro LC. Bone targeted therapy for advanced androgen.independent carcinoma of the prostate: a randomized phase II trial. Lancet 2001; 357: 336–41
- Lee N, Fawaaz R, Olsson CA, Benson MC, Petrylak DP, Schiff PB, et al. Which patients with newly diagnosed prostate cancer need a radionuclide bone scan? An analysis based on 631 patients. Int J Radiat Oncol Biol Phys 2000; 48: 1443–6
- Daniell HW. Osteoporosis after orchiectomy for prostate cancer. J Urol 1997; 157: 439
- Shahinian V, Kuo Y-F, Freeman J, Goodwin J. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 2005; 352: 154–64
- Oeflein MG, Ricchiuti M, Conrad W, Resnick MI. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. Am J Urol Rev 2004; 2(Suppl 2)16–20
- Tamada D, Sone T, Tomomitsu T, Jo Y, Tanaka H, Fukunaga M. Biochemical markers for the detection of bone metastatses of patients with prostate cancer: diagnostic efficacy and the effect of hormonal therapy. J Bone Miner Metab 2001; 19: 45–51
- Morote J, M'hammed YI, Martinez E, Esquena S, Lorente JA, Gelabert A. Increase of bone alkaline phosphatase after androgen deprivation therapy in patients with prostate cancer. Urology 2002; 59: 277–80
- Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer 2005; 103: 237–41
- Green JR. Skeletal complications of Prostate Cancer: Pathophysiology and Therapeutic Potential of Bisphosphonates. Acta Oncol 2005; 44: 284–94