We report a case of an elderly gentleman with a large metastatic hepatocellular carcinoma (HCC) who was successfully treated with a combination of oral capecitabine, megestrol and thalidomide.
Our patient is a 73 year-old-Chinese male who first presented in January 1999, at age 67, with acute right hypochondrial pain. Computer tomography (CT) scan of the abdomen revealed a large 7 cm×7.5 cm hypervascular tumor situated in segments 5, 7 and 8 of the right hepatic lobe. Abdominal angiography confirms the lipiodol-avid nature of this tumor mass. Alpha-feto protein (αFP) was marginally elevated at 48.9 ug/l. The patient was negative for Hepatitis B and C virus and denied alcohol abuse. He had an ECOG performance status of 0 and classified under Child-Pugh class A.
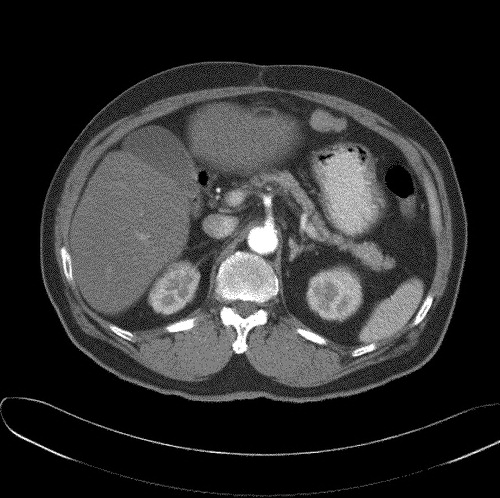
He underwent surgical resection. Histology confirmed the presence of a large 10 cm HCC, with close resection margins of less than 0.5 mm. Macroscopically the tumor demonstrated capsular invasion. Post-operatively our patient received concurrent chemo-radiation with conformal radiotherapy (RT), 50.6 Gy over 28 fractions, with bolus 5-fluorouracil and leucovorin on weeks one and five of RT. He remained disease free until December 2002 when a routine CT abdomen () revealed a new peritoneal nodule measuring 1.8 cm×3.3 cm suspicious of a peritoneal metastasis. The liver was radiologically unremarkable. There was no clinical evidence of a second malignancy.
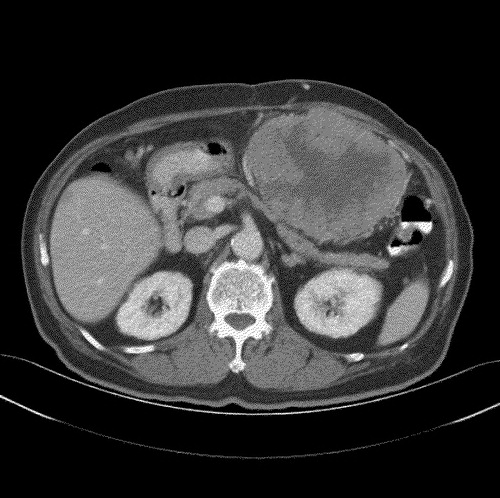
The αFP level was 4.6 ug/l. As the patient declined further surgical or medical intervention, he was managed expectantly. However, the mass continued to increase in size, and by November 2003 (), it had enlarged to 12.3 cm×9.4 cm. The patient finally agreed to a trial of chemotherapy, albeit oral only, and was started on a combination of oral capecitabine 2 g twice daily for 14 days on a 21 day cycle (approximately 2200 mg/m2), with thalidomide 150 mg and megestrol acetate 160 mg once daily.
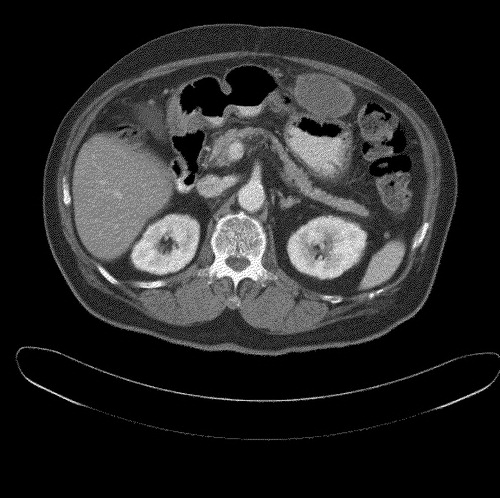
Interval CT evaluations revealed significant reduction in tumor bulk to 7.5 cm×5.2 cm and 5.1 cm×4.2 cm after three and five cycles of chemotherapy, respectively. He was given a total of 13 cycles of chemotherapy. CT evaluation in September 2004 () showed continued radiological response, with the mass measuring only 4.9 cm×3.3 cm. No new lesions were noted.
In view of the excellent tumor response and the absence of new sites of metastases, resection of the residual peritoneal mass was performed. Histological examination revealed no viable tumor. The patient thus achieved a pathological complete response (CR). He was last evaluated in May 2005 and remained in complete clinical remission.
The toxicities encountered were generally tolerable and minor with no significant grade three or four haematological or non-haematological toxicities encountered.
Discussion
This is the first reported case using a combination of oral capecitabine, megestrol acetate and thalidomide in advanced HCC, and resulting in a pathological complete response.
Hepatocellular carcinoma (HCC) is one of the commonest malignancies in Asia and is the third most common cancer among men in Singapore Citation[1]. It is a highly aggressive tumor, often diagnosed late in the course of disease, chemo-refractory and frequently occurs in the setting of chronic liver disease and advanced cirrhosis. As such, the treatment options are limited.
There is currently no standard recommended treatment for advanced HCC. Systemic agents that have been shown to have some activity in HCC, include chemotherapeutic agents such as doxorubicin, cisplatin, 5-fluorouracil and newer agents like gemcitabine, and capecitabine. Objective response rates (RR) to these single agents are low. Combination chemotherapy has not been shown conclusively to be superior to single agent chemotherapy and is associated with higher rates of treatment related toxicities. Apart from chemotherapy, immunotherapeutic agents such as interferon-alfa, hormonal therapy and thalidomide have also been investigated in patients with HCC with mixed results. There is currently little information on the use of these agents in combination with chemotherapy in patients with HCC.
Combining capecitabine, megestrol acetate and thalidomide is rational. Individually, each drug has been reported to possess modest anti-tumor activity in HCC. Response rates to single agent capecitabine Citation[2] and thalidomide Citation[3] in small phase II studies ranged from range from 6.7% to 11%, while megestrol acetate has been shown to improve survival in one study Citation[4]. Additionally, these drugs may be potentially synergistic as they act via different mechanisms. Capectabine is an oral fluoropyrimidine with broad anti-tumor activity in solid tumors. It is converted to 5-flourauracil within the cancer tumor cells where it functions as an anti-metabolite. While the precise mechanism of action of megestrol acetate is uncertain, it is postulated to act through estrogen receptors, which have been demonstrated to be present in up to one-third of patients with HCC Citation[5]. Being a highly vascular tumor, HCC is theoretically susceptible to anti-angiogenic agents such as thalidomide, which has been shown to target vascular endothelial growth factor (VEGF) and basic-fibroblastic growth factor-alpha. Apart from their different mechanisms of action, the combined use of these agents is also attractive as they are generally well tolerated with non-overlapping toxicities and offers the convenience of oral administration.
While the role of metastacectomy in HCC is not established, based on the long progression-free interval, absence of new systemic lesions over time and excellent clinical response to combination therapy, we decided to proceed with resection of the residual peritoneal mass. At the time of this writing, eight months after the surgery, our patient remains clinically free from disease.
In summary, we report a case of a pathological complete response, in a patient with previously treated, metastatic HCC, to a novel combination of oral capecitabine, megestrol and thalidomide. In view of its efficacy, low toxicities and ease of administration, this drug regimen should be further evaluated
References
- Seow, A, Koh, WP, Chia, KS, Shi, LM, Lee, HP, Shanmugaratnam, K. Trends in cancer incidence in Singapore 1968–2002, Singapore Cancer Registry Report No.6.
- Patt YZ, Hassan MM, Aguayo A, Nooka AK, Lozano RD, Curley SA, et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallblader carcinoma. Cancer 2004; 101: 578–86
- Hsu C, Chen CN, Chen LT, Wu CY, Yang PM, Lai MY, et al. Low–dose thalidomide treatment for advanced hepatocellular carcinoma. Oncology 2003; 65: 242–9
- Villa E, Ferretti I, Grottola A, Buttafoco P, Buono MG, Giannini F, et al. Hormonal therapy with megestrol in inoperable hepatocellular carcinoma characterized by variant oestrogen receptors. Br J Cancer 2001; 84: 881–5
- Engstrom PF, Levin B, Moertel CG, Schutt A. A phase II trial of tamoxifen in hepatocellular carcinoma. Cancer 1990; 65: 2641–3