Esophageal cancer has a low incidence in the western world. About 300 to 400 new cases occur in Sweden annually, but the incidence, especially of distal adenocarcinoma, is increasing. Even with radical surgery or definitive radiotherapy, 5-year survival is poor (<10%) Citation[1]. When the diagnosis is made, approximately 50% of patients are not candidates for curative therapy due to locally advanced disease or metastases. In this group of patients, median survival time is 6 months or less with or without palliative treatment Citation[2]. To date, the treatment of choice in advanced esophageal carcinoma has been cisplatin and 5-FU with concomitant radiotherapy Citation[3], Citation[4]. A second-generation platinum analogue, oxaliplatin, seems to have comparable or possibly even better therapeutic effect with substantially fewer side effects Citation[5]. In this study, we report our initial experience with the use of oxaliplatin in advanced esophageal carcinoma.
The study included twenty consecutive patients (8 women, 12 men) with locally advanced esophageal carcinoma patients at the Department of Oncology, University Hospital of Malmö, Sweden between June 2000 and April 2002. Diagnosis and staging were established through endoscopy with biopsies, thoracoabdominal computerized tomography and endoscopic ultrasound. Mean age was 64 years (range 46–85). One patient had stage II, 12 patients had stage III, and 7 patients had stage IV disease. Squamous cell carcinoma was seen in 8 patients, adenocarcinoma in 11 patients, and poorly differentiated carcinoma in one patient.
Chemoradiotherapy was given on an outpatient basis. Before treatment, IV access (Port-A-Cath) and a percutaneous or surgical gastrostomy were carried out in all patients. Hemoglobin, leukocytes, platelets, electrolytes, creatinine, and liver function tests were checked before each course of chemotherapy.
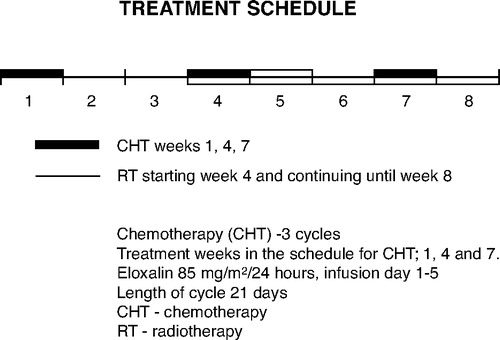
The patients received chemotherapy for eight weeks as three 5-day courses with oxaliplatin and 5-FU (). On day 1, oxaliplatin 85 mg/m2 was administered for 30 to 120 minutes in the outpatient clinic. During days 1–5, the patient received 5-fluorouracil 750 mg/m2 at home by means of a portable pump. Five weeks of concomitant radiotherapy (25×2 Gy) was started on an outpatient basis along with the second course of chemotherapy (week 3). A special body support was used to immobilize the patient, both during pretreatment CT-scanning and during treatment sessions. Each patient received an individually, computerized dose from an 8 mV linear accelerator. Treatment evaluation according to WHO-criteria were done every 4 to 8 weeks until death or October 2004. During follow-up, we always carried out clinical examination and blood chemistries, while endoscopy, endoscopic ultrasound, and computerized tomography were done as indicated. Two patients received only Oxaliplatin on a weekly basis concomitant with external radiation for a total radiation dose of 40 Gy.
Results
Treatment was generally well tolerated and safe. All treatments were given on an outpatient basis, and no patient was hospitalized for toxic side effects. No dose reductions were necessary for grade III to IV toxicity, and no cardiac toxicity was observed. Massive gastrointestinal bleeding occurred in two patients. Although this is a common complication in advanced esophageal carcinoma, a contributory effect from the chemoradiotherapy cannot be completely ruled out.
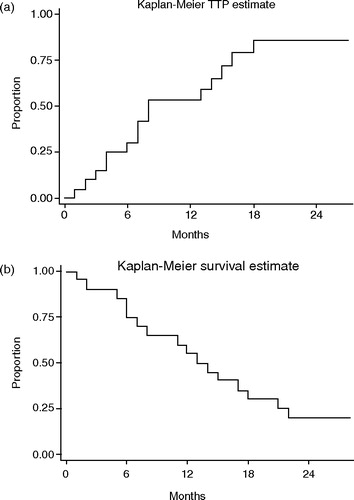
Progression of the tumor during treatment was seen in four patients, while sixteen (80%) patients responded to treatment. In six patients, a complete disappearance of all tumor was seen, and in ten patients more than a 50%-reduction of tumor. Median time to progression (TTP) was nine months (). At follow-up, four patients were free from tumor and alive NED, and two patients were alive with disease. Fourteen patients died, ten from tumor progression and four from other causes. Of particular interest is one patient, aged 80 years, where treatment led to a complete regression of radiographically visible tumor. He tolerated the outpatient treatment well without complications. Two months after treatment, he was admitted to the hospital for fever, cough, and diffuse illness. A chest X-ray showed suspected pulmonary metastases. Palliative corticosteroid treatment resulted in prompt disappearance of both symptoms and radiographic changes. The clinical picture was reinterpreted as pneumonitis, and further treatment led to complete resolution without sequelae. The patient subsequently died without any signs of recurrent disease.
There is controversy as to whether initial treatment for locally advanced inoperable esophageal tumor should be palliative or curative. Even when the patient is considered incurable from a surgical perspective, we see increasing evidence that patients may be cured with nonsurgical therapy Citation[4]. Earlier studies have shown that the combination of platinum-based chemotherapy with radiation has resulted in complete tumor regression in both phase II and randomized studies. Therefore, cisplatin-based chemotherapy has been standard treatment in advanced esophageal carcinoma. Many of these patients are in a catabolic state with weight loss and dehydration, and are at high risk for toxic side effects. In fact, considerable toxicity (e.g. nephrotoxicity, ototoxicity, and cardiac toxicity) has been reported with cisplatin Citation[6]. During treatment, renal function and diuresis need to be carefully monitored, and rehydration, forced diuresis, and mannitol treatment may be indicated. In contrast, oxaliplatin is considerably less toxic, and there is less need for monitoring. Moreover it can be given to patients in situations where cisplatin is considered unsuitable because of reduced hearing, reduced kidney function, or advanced age. In this study, chemoradiotherapy with oxaliplatin and 5-FU was well tolerated and all treatment was given on an outpatient basis.
The antitumoral and radiosensitizing effects of oxaliplatin seem at least comparable with cisplatin in the treatment of esophageal carcinoma Citation[5]. In the present study, 80% of the patients responded to treatment for a median time of 9 months, which we find encouraging.
References
- Muller JM, Erasmi H, Stelzner M, Zieren U, Pichlmaier H. Surgical therapy of oesophageal carcinoma. Br J Surg 1990; 77(8)845–57
- Albertsson M, Ewers SB, Widmark H, Hambraeus G, Lillo-Gil R, Ranstam J. Evaluation of the palliative effect of radiotherapy for esophageal carcinoma. Acta Oncol 1989; 28(2)267–70
- Albertsson M. Chemoradiotherapy of esophageal cancer. Acta Oncol 2002; 41(2)118–23
- Ask A, Albertsson M, Jarhult J, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in oesophageal cancer. Acta Oncol 2003; 42(5–6)462–75
- Leichman L, Pendyala L, Leichman CG. Definitive and neoadjuvant therapies for esophageal and gastroesophageal junction tumors: a look back and toward the future. Semin Oncol 2003; 30(4 Suppl 11)11–8
- Cwikiel M, Albertsson M, Hambraeus G. Acute and delayed effects of radiotherapy in patients with oesophageal squamous cell carcinoma treated with chemotherapy, surgery and pre- and postoperative radiotherapy. Acta Oncol 1994; 33(1)49–53