Abstract
The etiology of non-Hodgkin lymphoma, as well as its global dramatic rise in incidence during the past decades, remains largely unexplained. However, there is increasing awareness that this group of malignancies may entail not only clinical, morphological and molecular heterogeneity, but also considerable variations in terms of etiologic factors. In this review, epidemiologic patterns are summarized as well as current evidence of associations between various known or suspected risk factors for non-Hodgkin lymphoma overall or for any of its subtypes. Central pathogenetic mechanisms include immunosuppression, especially in relation to T-cell function and loss of control of latent EBV infection, and chronic antigen stimulation. Some degree of familiar aggregation also implies a role for genetic susceptibility. A number of recent investigations of non-Hodgkin lymphoma etiology will hopefully lead to a better understanding of the causes of these malignancies.
The malignant lymphomas entail considerable heterogeneity with regard to morphological and molecular characteristics as well as clinical course Citation[1]. The non-Hodgkin lymphomas (NHL) make up around 90%, and Hodgkin lymphoma (HL) account for the remaining 10% percent of all malignant lymphomas. NHL arises, with few exceptions, from two distinct lymphocyte types, B or T cells, and the heterogeneity is at least in part related to the many stages of normal differentiation and maturation of these cells Citation[1]. A dramatic rise in incidence of NHL worldwide during the past decades, surpassed only by lung cancer in women and malignant melanoma in both sexes Citation[2], has sparked intense research efforts to reveal the causes of this group of malignancies as well as to explain the past increase. Although some strong risk factors have been identified (including primary immunodeficiency disorders, HIV-infection and organ transplantation), the causal factors behind most newly diagnosed NHL patients remain unknown. However, it has become clear that also etiologic variation among NHL subtypes may be considerable Citation[2]. The aim of this review is to describe the epidemiology of NHL, to summarize current evidence of associations with an array of known and possible etiologic factors, and to outline established and potential biological mechanisms of lymphomagenesis. In addition, there is a brief description of a recent large Scandinavian initiative to investigate risk factors of malignant lymphomas.
Descriptive epidemiology
Classification
NHL is presently classified according to the universally accepted World Health Organization (WHO) classification where 36 subtypes (21 of B-cell and 15 of T-cell type) are recognised, excluding entities of uncertain malignant potential Citation[1]. Chronic lymphocytic leukemia (CLL) now belongs to the NHL group, together with its non-leukemic counterpart small lymphocytic lymphoma Citation[1]. Plasma cell malignancies are now also recognised as NHL subtypes according to WHO. This review focuses on NHL excluding CLL and plasma cell malignancies.
The variation in clinical presentation and course, treatment approaches and prognosis among NHL subtypes is considerable, to say the least. In spite of major advances in the understanding of lymphoma biology reflected in the WHO classification, a clinical subdivision into indolent, aggressive and very aggressive lymphoma types is still meaningful. Other aspects considered in the clinical setting are location and disease spread. The International Prognostic Index, published in 1993 Citation[3] (based on age, performance status, serum lactate dehydrogenase, number of involved extranodal sites, Ann Arbor stage) separates patients into clinically distinct groups with varying prognoses. Newer technologies, such as cDNA microarrays, have further distinguished patients into distinct prognostic groups even within histologic subtypes. For example, there appears to be at least two subcategories of diffuse large B-cell lymphoma, a germinal center B-cell type and a less favorable activated B-cell type based on differences in gene expression profiling Citation[4]. Thus, the lymphoma classification schemes currently in use will almost certainly be subject to further refinement in the future.
Incidence
NHL (excluding CLL and plasma cell malignancies) is more common in the developed world, with the highest incidence rates in the USA, Australia and New Zealand, and Europe, and the lowest in Eastern and South Central Asia Citation[5]. Around the year 2000, the age standardized (world standard) incidence of NHL was estimated at approximately 14 per 100 000 person-years in the USA and Canada, 10 per 100 000 in Denmark and Sweden, and 3 per 100 000 in South Central Asia Citation[5]. However, the rare T-cell neoplasms are more common in Asia than in other regions. Worldwide, NHL constitutes the tenth most commonly diagnosed malignancy, whereas in the developed world it ranks seven. In Sweden in 2003, malignant lymphomas (NHL and HL) were the eighth most common new cancer diagnoses among males and the tenth most common in females Citation[6]. In the USA, NHL has climbed to the fifth most frequently diagnosed malignancy in recent years Citation[7]. The most common NHL subtypes by far in developed countries (disregarding CLL and plasma cell entities) are diffuse large B-cell lymphoma (about 30%) and follicular lymphoma (about 20%). All other NHL subtypes have a frequency of less than 10% Citation[1]. Many subtypes are characterised by a slight preponderance of males, most striking in mantle cell lymphoma (70% males), whereas females predominate in follicular lymphoma Citation[1].
Time trends
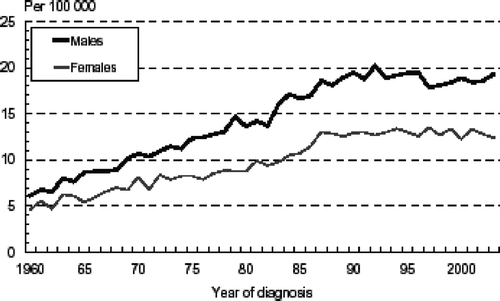
For several decades, there has been a dramatic increase in NHL incidence worldwide, of about 2–4% annually Citation[8]. The highest increase was observed in Denmark, where the rate doubled between 1970 and 1985 Citation[9]. In Scandinavia in general the increase in incidence was apparent already in the 1950s Citation[10], and in Connecticut, USA, even in the 1930s Citation[11]. Interestingly, this increase is not limited to developed countries, but has been observed also in for example India, Japan, Brazil, Singapore and Puerto Rico Citation[12]. In the beginning of the 1990s, the rise in incidence began to level off in Sweden () Citation[6], and Denmark Citation[13]. In the USA, data from the Surveillance, Epidemiology, and End Results (SEER) Program showed stabilization in overall NHL incidence rates in the early 1990s and then subsequent decline. However, in population groups at low risk of human immunodeficiency virus (HIV)-infection and acquired immunodeficiency syndrome (AIDS), such as men above the age of 55 years and women of all ages, rate increases were still evident through the 1990s Citation[14].
Figure 1. Non-Hodgkin lymphoma (NHL) incidence in Sweden 1960 to 2003 in males and females, age-standardized to the Swedish population in the year 2000 (ICD: 200).
Less is known about time trends for NHL subtypes. Available information comes from studies using USA SEER data Citation[15]. Thus, between 1974 and 1992, a rapid increase was noted for diffuse large cell lymphoma, partly fuelled by the AIDS epidemic. More modest risk increases were observed for precursor lymphoblastic leukemia/lymphoma, Burkitt's or Burkitt-like lymphoma and follicular lymphoma, whereas no change was noted for CLL/small lymphocytic lymphoma or lymphoplasmacytic lymphoma. For several subtypes such as the mantle cell and marginal zone lymphomas, time trends are difficult to assess due to the recent recognition of these entities as distinct subtypes. Concerning the group of T-cell lymphomas, SEER data have shown an increase of mycosis fungoides, but otherwise epidemiological data are scarce Citation[15]. Primary extranodal lymphoma, particularly located in the central nervous system, has increased more rapidly than nodal forms since the 1970s Citation[12]. However, a decline in rates of central nervous system lymphomas has been noted since the mid-1990s in the USA, in parallel with the decreasing incidence of AIDS Citation[2].
In 1992, an assembly was organized by the USA National Cancer Institute to evaluate the time trends of NHL. At this meeting, it was concluded that the increasing temporal trend was indeed real Citation[16], and that known and suspected risk factors could not explain the observed increase over time Citation[17]. Thus, after accounting for the likely effects of misclassification of diagnoses, the inclusion of new entities of NHL, and for established risk factors (see below), it was estimated that close to 50% of the observed increase in both sexes remained unexplained Citation[17]. In view of the absence of major breakthroughs concerning the etiology of NHL since then (with the exception of the role of a few infectious agents in specific lymphoma forms, most notably Helicobacter pylori in gastric lymphomas, however representing few cases in the population), the conclusions from the 1992 meeting appear still valid.
Etiology of non-Hodgkin lymphoma
Pathogenesis
B and T lymphocytes are important members of the immune system that above all serve to protect against infectious agents Citation[18]. In general, B cells produce antibodies with antigen-binding capacity, whereas T cells recognize antigen presented by other cells. A variety of different secreted proteins, or cytokines, released by activated T cells (especially of the T helper cell, or CD4+, type) serve to alert and coordinate the local immune response. In light of the importance of the T cells in controlling B-cell as well as overall immune function, it is perhaps not surprising that the strongest and most well-established risk factors for malignant lymphomas are characterized by dysregulation or suppression of T-cell function (HIV/AIDS, organ transplantation, see below) that allow for Epstein-Barr virus (EBV)-driven B-cell proliferation and transformation.
As in cancer development in general, neoplastic transformation of T or B cells represents a multistep process with progressive accumulation of genetic lesions that result in clonal expansion and establishment of a solid or leukemic tumor. Mechanisms may involve dysregulation of cell growth, cell signaling pathways and programmed cell death (apoptosis). The intricate rearrangements in B-cell immunoglobulin or T-cell receptor genes during the normal differentiation and adaptation of these cells represent genetically vulnerable stages. During these processes, physiologically occurring DNA double-strand breaks pave the way for aberrant chromosomal translocations, which are typical of NHL tumors. In fact, chromosomal translocations have been observed in up to 90% of NHL cases Citation[19], Citation[20]. These translocations, with or without additional genetic lesions, can precipitate the activation of oncogenes or inactivation of tumor suppressor genes Citation[1]. Oncogenic viruses provide other possible mechanisms for genetic lesions, as well as direct carcinogenesis by environmental factors. Although the importance of genetic factors in lymphoma development is evident, the geographically uniform rise in NHL incidence implicates a crucial role of one or several environmental agents in the etiology of NHL.
Immunosuppression
Either primary or genetic disorders of immune dysfunction and acquired states of severe immunosuppression (HIV/AIDS and organ transplantation) constitute strong and well-established risk factors for NHL, but explain few new cases in the general population. Clinical features shared by the majority of immunodeficiency-related lymphomas are diffuse large B-cell histology, extra-nodal location especially in the gastrointestinal tract and central nervous system, aggressive clinical course, and an association with EBV. It has been suggested that minor degrees of immunodeficiency also may mediate the development of lymphoma. However, judging from lymphomas occurring in HIV/AIDS and after organ transplantation, it would be predicted that EBV-driven lymphoma most often would be the result. As most lymphomas occurring in the population are not EBV-positive, the HIV/AIDS and post-transplantation settings may not offer ideal causal models for the study of lymphoma etiology in the general population Citation[21].
Primary disorders of immune dysfunction
Inherited disorders most commonly associated with an excess risk of NHL include ataxia-telangiectasia, Wiskott-Aldrich syndrome, common variable immunodeficiency, severe combined immunodeficiency, X-linked lymphoproliferative disorder, Nijmegen breakage syndrome, hyper-IgM syndrome and autoimmune lymphoproliferative syndrome Citation[1]. It has been estimated that up to 25% of patients with these disorders will develop tumors, primarily B-cell lymphoma, during their lifetime and often already in childhood Citation[22]. The mechanisms of lymphomagenesis are related to the underlying disorder and involve loss of T-cell control (Wiskott-Aldrich syndrome), apoptosis defects (autoimmune lymphoproliferative syndrome), abnormal DNA repair function (ataxia-telangiectasia, Nijmegen breakage syndrome), defective T-cell/B-cell interactions (hyper-IgM-syndrome), and perhaps chronic antigen stimulation (common variable immune deficiency) Citation[1]. Defective immune surveillance of EBV infection is an important co-factor in lymphoma development in these cases.
HIV/AIDS
Overall NHL risk has been estimated to be increased about 60- to 200-fold in HIV positive individuals relative to the general population, with low-range risk increases for indolent NHL types and higher excess risk for diffuse large cell NHL, in particular of the immunoblastic type Citation[23]. A decline in the incidence of AIDS-related lymphomas has been noted since the introduction of highly active antiretroviral therapy (HAART) Citation[24], nevertheless, NHL still accounts for more than 20% of AIDS-related deaths in developed countries Citation[23]. The classic model of HIV-associated lymphomagenesis proposes chronic antigenic stimulation of B lymphocytes and macrophages induced mainly by EBV, but also human herpes virus 8 (HHV-8), in the presence of disturbed T-cell function and low CD4 cell counts. However, nearly 50% of AIDS-related lymphomas are negative for EBV or HHV8. Therefore, other factors must also influence HIV-related lymphomagenesis such as genetic abnormalities, cytokine dysregulation and dendritic cell impairment induced by HIV itself Citation[23]. Although HIV is not characterized by typical oncogenic potential by insertional mutagenesis, occasional non-random integration of the viral genome could possibly contribute to oncogenic transformation in a subset of patients Citation[25].
Organ transplantation
A greatly increased relative risk of NHL in conjunction with potent immunosuppressive therapy following renal, liver, heart or bone marrow transplantation has been reported consistently Citation[8]. The relative risk of malignant lymphomas increases by about 10- to 20-fold in renal allograft recipients and up to about 200-fold in heart transplant recipients, compared to the general population Citation[26]. Post-transplant lymphoproliferative disorders comprise a spectrum ranging from early EBV-driven polyclonal proliferations to EBV-positive (80–90%) or EBV-negative malignant lymphomas, predominantly of B-cell origin Citation[27]. EBV-negative cases typically occur later than EBV-positive tumors: the majority of cases occurring more than five years after transplantation are EBV-negative. The pathogenesis of post-transplant lymphoproliferative disorders is most likely complex and multifactorial although drug-induced impaired T-cell immune surveillance in combination with chronic antigenic stimulation exerted by the graft is believed to have a central role Citation[27]. Modulating factors include donor and recipient EBV serologic status, type of transplanted organ, underlying disease and type, duration and intensity of the immunosuppressive treatment Citation[27].
Other genetic factors
Familial aggregation of hematopoietic malignancies has been consistently reported, thus implicating a role of genetic susceptibility in lymphoma development. The risk of NHL is increased about 2- to 3-fold in first-degree relatives of patients with lymphoma or hematopoietic cancer Citation[28–32]. Higher excess risks of about 7-fold or more have been reported for CLL in first-degree relatives of CLL patients Citation[33]. It cannot be excluded, however, that familial clustering is attributable to shared environmental exposures rather than genetic predisposition. A few studies have described a tendency towards differential lymphoma risks by type of first-degree relation Citation[29], Citation[31], Citation[32], i.e., higher risk in siblings compared to parents and offspring of index persons with lymphoma or hematopoetic cancer. This may indicate that both shared genetic and environmental factors contribute to the observed overall association.
In recent years, several studies of common genetic variation that could confer susceptibility to malignant lymphomas in the general population have been published. Examples of specific genes investigated include those encoding for tumor necrosis factor alpha Citation[34] and other cytokines Citation[35], the p53 protein Citation[36], DNA repair proteins Citation[37], biotransformation enzymes Citation[38], intermediates of the folate metabolism pathway Citation[39], human leukocyte antigens (HLA) Citation[40], and transcriptional factors (Bcl6 Citation[41]). However, no strong susceptibility loci have yet been confirmed.
Infectious agents
Infectious agents consistently associated with malignant lymphomas include the herpesviruses EBV and HHV-8, and the retrovirus human T-cell lymphotrophic virus 1 (HTLV-1). More recent evidence also suggests a role for the hepatitis C virus (HCV), a single-strand RNA virus, whereas the role of simian virus 40 (SV40) of the polyoma virus family remains uncertain. Associated bacteria include Helicobacter pylori (H. pylori) and perhaps also Borrelia burgdorferi.
EBV
Widespread in all human populations, EBV persists in the vast majority of individuals as a lifelong asymptomatic infection of the B-lymphocyte pool. Primary infection with EBV usually occurs in childhood, but if it is delayed until adolescence it presents as infectious mononucleosis in about half of those infected Citation[42]. In healthy individuals, equilibrium exists between latent EBV infection and the host's immune system, where continued T-cell surveillance is of special importance. However, with severe immunodeficiency, control mechanisms are impaired which may lead to EBV-driven B-cell proliferation and development of B-cell lymphoma Citation[43]. Importantly, EBV-encoded latent genes are capable of transforming B lymphocytes in vitro by altering cellular gene transcription and key cell signaling processes Citation[44]. Apart from a strong association with lymphomas in immuno-compromised hosts, early EBV infection is also associated with Burkitt lymphoma in Africa but infrequently in other parts of the world, and with a few rare but specific types of T- and NK-cell lymphomas (predominantly occurring in Asia). Occasionally, EBV DNA has been found in B-cell tumors other than Burkitt lymphoma in immunocompetent hosts, but only to a small extent (less than 5%), and without evidence of linkage to a specific B-cell NHL type Citation[45].
HHV-8
Also called Kaposi sarcoma herpes virus (KSHV), HHV-8 is detected in the majority of primary effusion or body cavity lymphomas. This rare lymphoma type occurs almost exclusively in HIV-infected individuals, but can develop occasionally in the absence of immunodeficiency in areas of high HHV-8 seroprevalence, such as the Mediterranean. Because patients with primary effusion lymphoma are often coinfected with EBV, the delineation of the etiologic role of each virus is difficult Citation[45]. However, observations of tumors being monoclonal expansions of a single infected cell support an etiologic role of HHV-8 Citation[46].
HTLV-1
This human retrovirus is causally associated with adult T-cell leukemia/lymphoma in the Caribbean and Japan, where infection is endemic. The virus causes a latent persistent infection in circulating T lymphocytes. Adult T-cell leukemia/lymphoma develops in 2 to 5% of HTLV-1 infected individuals after a long latent period, suggesting a multistage process of T-cell transformation and involvement of additional pathogenetic factors Citation[47]. HTLV-1 infection is rare in Europe and the USA, but some studies from the USA have indicated a possible association between HTLV-1 and mycosis fungoides or Sézary syndrome Citation[1].
HCV
Investigators have reported 2- to 14-fold increased relative risk of B-cell NHL in association with hepatitis C infection, but results are inconsistent Citation[48], Citation[49]. Positive associations are mostly reported from geographical areas with high HCV seroprevalence, such as southern and eastern Europe, Japan and southern USA, whereas no associations have generally been noted in studies from central and northern Europe, northern USA or Canada Citation[49]. Thus, it has been estimated that the proportion of B-cell lymphoma cases potentially attributable to HCV infection in countries with a high seroprevalence is about 5 to 10%, but much lower if at all existent in other countries Citation[49], Citation[50]. HCV is both lympho- and hepatotrophic and replicates in mononuclear blood cells. A specific HCV protein (E2) may be responsible for chronic antigen-driven polyclonal B-cell proliferation which may lead to lymphoma development in the presence of unidentified genetic or environmental cofactors Citation[51]. Interestingly, case reports suggest that low-grade B-cell lymphomas associated with HCV infection may regress after successful antiviral and interferon therapy Citation[52]. Mixed cryoglobulinemia, a vasculitic immune complex disorder, develops in a minority of HCV infected individuals and is frequently associated with benign lymphoproliferations in the liver and bone marrow. Overt malignant lymphoma occurs in about 10% of these cases Citation[53].
SV40
The monkey polyoma virus SV40 is known to induce a variety of cancer types in laboratory animals, including lymphomas, by inactivation of the tumor suppressor genes p53 and pRBCitation[54]. This virus accidently contaminated the Salk polio vaccine administered during the years 1955 to 1962 Citation[55]. However, the infection also exists among recipients of non-contaminated vaccines Citation[56]. In a few studies in humans, SV40-specific DNA sequences have been detected in a higher proportion in NHL tumor tissue than in control samples of normal lymphoid tissue or other tumors Citation[57–59], but several other studies have failed to confirm viral presence in lymphoma lesions Citation[60–64]. Furthermore, serum levels of SV40 antibodies have not been associated with lymphoma risk Citation[55], Citation[65]. Also, comparisons of lymphoma risk in recipients of contaminated versus uncontaminated polio vaccine support no association Citation[66], Citation[67]. In conclusion, available evidence for a role of SV40 in NHL etiology is weak.
Helicobacter pylori
This gastric pathogen causes chronic gastritis, and its association with primary gastric lymphoma of the low-grade MALT-type is well established. In one of the first and most important studies, H. pylori infection in gastric tissue was detected in over 90% of cases with gastric MALT lymphoma Citation[68]. The relative risk of gastric MALT lymphoma has been estimated to be increased about six-fold in association with serologic evidence of H. pylori infection Citation[69]. In vitro studies have shown that B-cell proliferation in response to H. pylori is mediated by tumor-infiltrating T cells Citation[70]. Clinical trials further support a causal link, as about 75% of gastric MALT lymphomas regress upon eradication of H. pylori with antibiotic treatment Citation[71].
Other pathogens
Infection with Borrelia burgdorferi has been associated with the development of primary cutaneous B-cell lymphoma in studies from European countries Citation[72]. However, no association has been observed in North America Citation[73]. The discrepancy may be due to genetic and phenotypic differences between Borrelia burgdorferi strains in Europe and the USA Citation[73]. A bacterial etiology is also suspected for the uncommon immunoproliferative small intestinal disease (also known as alpha chain disease) arising from small intestinal mucosa-associated lymphoid tissue (MALT). Early-stage lesions may respond to antibiotic treatment, and isolation of Campylobacter jejunei from tumor tissue was recently reported in a small case series Citation[74]. Chlamydia psittaci has been linked to ocular adnexal lymphomas Citation[75]. In addition, a number of chronic infectious disorders including tuberculosis, malaria, pyelonephritis and herpes zoster have been associated with increased risk of NHL overall in epidemiological studies Citation[76–79]. Hence, the number of specific infectious agents found to be associated with lymphoproliferative malignancies is clearly growing, consistent with the idea of chronic immune stimulation as a potential risk factor for lymphomagenesis.
Autoimmune and chronic inflammatory disorders
Excess risks of malignant lymphomas have been consistently reported in rheumatoid arthritis (RA), Sjögren's syndrome, systemic lupus erythematosus (SLE), celiac disease, dermatitis herpetiformis, poly- and dermatomyositis, and chronic thyroiditis Citation[80]. Other autoimmune and chronic inflammatory disorders, such as psoriasis Citation[81], Citation[82] and inflammatory bowel disorders Citation[83–85], have occasionally, but not invariably, been associated with increased lymphoma risks. Spurious positive associations could arise in this context due to misclassification, as lymphomas may mimic inflammatory disorders and be accompanied by autoimmune paraneoplastic phenomena Citation[86].
Most well-documented is the association with NHL in patients with Sjögren's syndrome. Reported increases in relative risk range from 4.5- to 44-fold, with higher estimates for primary disease than when secondary to RA, SLE or myositis Citation[80], Citation[87], Citation[88]. More severe disease characterized by parotid enlargement, hypocomplementemia and low CD4 cell counts has been associated with accentuated NHL risks Citation[89], Citation[90], but no association has been reported with immunosuppressive treatment Citation[80]. Case reports and case series have indicated a strong association with MALT lymphomas, especially in the parotid gland Citation[91], Citation[92], but the risk of diffuse large B-cell lymphoma may also be increased Citation[90], Citation[93]. Local pathogenesis in affected glandular tissue involves chronic inflammation and T-cell-dependent antigen stimulation. If these mechanisms are responsible also for development of non-organ specific lymphoma in Sjögren's syndrome is, however, not clear. A number of studies have also demonstrated increased risks of NHL in patients with RA Citation[94–99]. On average, reported excess risks of NHL overall range between 1.5- and 4-fold increased Citation[87], Citation[94–96]. The underlying reasons for the increased NHL risk in RA patients remain a matter of debate. Particular concern has been raised for treatment with immunosuppressants Citation[80], and more recently also for the biological tumor necrosis factor-blocking agents Citation[100]. In a few studies with information on markers of inflammatory activity as well as treatment Citation[93], Citation[97], Citation[101], degree of inflammation was however suggested to be more important than treatment. EBV has been detected at low frequency in RA-related lymphomas Citation[101], Citation[102] and is therefore not likely to be of major importance.
Skin cancer and ultraviolet radiation exposure
Numerous studies have described increased risks of HL, NHL and CLL following a diagnosis of skin cancer, including malignant melanoma, squamous cell carcinoma and basal cell carcinoma. Conversely, an increased risk of all three forms of skin cancer has been noted following a history of lymphoma Citation[103–108]. These observations, along with parallel time trends in incidence of skin cancer and NHL, gave rise to the former popular hypothesis that ultraviolet radiation exposure would increase risk, not only of skin cancer, but also NHL Citation[11], Citation[109]. However, two recent studies in different parts of the world contrarily observed inverse associations between frequent UV radiation exposure and risk of NHL Citation[110], Citation[111]. Thus, alternative explanatory mechanisms for the link between skin cancer and NHL needs to be considered, including common genetic susceptibility, post-treatment effects and/or detection bias Citation[106–108]. The surprising finding that UV radiation exposure may be inversely associated with risk of NHL Citation[110], Citation[111] first of all needs to be confirmed in additional studies. Possible mechanisms behind such an association include UV-induced systemic immune modulation Citation[112] or photo-activation of vitamin D-production Citation[113].
Allergy
Similar to the incidence of NHL, the incidence of allergic disorders has increased epidemically during the past decades. However, evidence suggests that several allergic conditions may be associated with a reduced risk of NHL. A decrease in risk has been observed repeatedly in case-control studies in individuals with allergic skin conditions or with a history of allergy to grass or pollen Citation[114–118], but the majority of these studies have used self-reported history of allergy, and the distinction between non-allergic conditions and allergy has not always been clear. Also, recent cohort studies with prospectively collected data on allergy could, not confirm a decreased lymphoma risk among these patients Citation[119], Citation[120]. Allergic reactions have been suggested to be related to a reduced NHL risk through promotion of B-cell differentiation Citation[121] and/or by skewing the T helper cell immune response towards increased T helper type 2 activity Citation[122].
Occupational exposures
An increased risk of NHL has been suggested in a variety of occupational groups, such as farmers, pesticide applicators, grain millers, wood and forestry workers and workers in the petroleum, rubber, plastic, and synthetics industries Citation[123]. Potentially hazardous exposures among these groups include pesticides (phenoxy acids, organophosphates, organochlorines and others), benzene and other organic solvents Citation[123]. A positive association between pesticide exposures and NHL and CLL has been observed repeatedly, but not consistently Citation[56], Citation[124–126]. However, pesticides include a large number of different substances, of which only some may be truly related to lymphoma risk Citation[125]. Concern has been raised especially for the herbicide 2,4-dichlorophenoxyacetic acid Citation[56]. However, in a review of the effects of this herbicide on cancer risk, Garabrant et al. concluded that epidemiological studies provide weak evidence that 2,4-dichlorophenoxyacetic acid is associated with NHL or HL, and that biologically plausible effect mechanisms are lacking Citation[127]. Although the fraction of agricultural workers is small and decreasing in the developed world, domestic use of pesticides is widespread and increasing Citation[2]. Therefore, even small risk increases could explain a significant number of NHL cases and thus, further investigations are indeed warranted Citation[2]. According to another recent hypothesis, the leveling off of NHL incidence rates in several countries in recent years could be related to the banning of various organochlorine substances during the 1970s and 1980s in these countries Citation[128].
Benzene is a well-known leukemogenic agent (primarily causing acute myeloid leukemia), but current evidence for a causal link with NHL or CLL is insufficient Citation[129]. Other organic solvents, such as for example trichlorethylene, may be associated with increased risks of NHL Citation[130], Citation[131], but study inferences are limited by low power or poor exposure assessment Citation[130]. Another chemical compound of concern is dioxin, which is listed as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC) Citation[132]. However, this designation has been criticized partly for being based on observations of increased risks of cancer overall rather than one or a few specific cancer forms Citation[133]. Some epidemiological studies have shown increased NHL risk in association with dioxin exposure, but available data are inconsistent Citation[133].
Tobacco smoking
Study results on the possible role of tobacco smoking in NHL etiology are conflicting. Most reports have shown no excess risk of NHL overall in tobacco or cigarette smokers Citation[134–137], but there are several exceptions Citation[138–140]. Some studies have suggested a specific association with risk of the common follicular NHL subtype Citation[139], Citation[141], Citation[142]. Tobacco smoking appears to induce the bcl-2 oncogene translocation (14;18) in peripheral lymphocytes of healthy individuals Citation[143]. This translocation is also present in the tumor tissue of 70–95% of follicular lymphomas Citation[1]. However, one study that specifically evaluated tobacco use and risk of t(14;18)-positive NHL failed to show any clear association Citation[144].
Alcohol and dietary factors
The role of alcohol in the development of NHL is uncertain. Previous studies of alcohol intake have shown no association, or increased or reduced risk of NHL overall Citation[145], Citation[146]. Because dietary habits in western countries have changed dramatically over time, the potential influence of diet on NHL risk is of interest. Several investigations have suggested that higher consumption of meat, especially red meat, and dairy products may be associated with increased risk of NHL, and that increased intake of vegetables and fruits may reduce NHL risk Citation[147–149]. Diet could influence NHL risk through changes in energy balance, through direct exposure to dietary carcinogens and anti-carcinogens or by modulating the immune system, although evidence for any biological mechanism is limited Citation[149].
Blood transfusion
Interest in blood transfusions and NHL arose after findings of a positive association in a prospective cohort study in 1993 Citation[150]. Blood transfusions may promote lymphomagenesis through transmission of oncogenic viruses, transfusion-associated immunosuppression and/or engraftment of lymphoma cells from a donor with subclinical lymphoma. Although several early studies identified an elevated risk of NHL associated with transfusion of blood products, subsequent and larger studies were unable to confirm this association Citation[151–153]. Also, one large cohort study with adjustment for possible confounding by transfusion indication, reported no association with type of blood product, amount of blood transfused or latency period and risk of NHL or CLL Citation[154]. Thus, although an association between NHL and blood transfusion is biologically plausible, epidemiologic evidence of an association is weak, at most.
Medication
The existing literature concerning use of different drugs and NHL risk is contradictory. Past studies have found a significantly elevated risk of lymphoma in association with use of antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs) and other analgesics, corticosteroids, histamine2-receptor antagonists, psychotropic drugs, anti-convulsants, estrogen replacement therapy, antidepressants or anti-anxiety drugs, amphetamines, and/or digitalis or digitoxin Citation[114], Citation[115], Citation[155]. Conversely, perhaps just as many studies have detected no or even an inverse association between risk of NHL and these same medications Citation[98], Citation[156–158]. As several diseases, including acquired immunosuppression, autoimmune disorders, allergies and infections also appear to be associated with NHL risk, it is difficult to determine whether apparent associations between medications and lymphoma risk are due to the effects of the medications themselves, or rather the underlying disorder. In a recent large Scandinavian case-control study, use of corticosteroids, histamine2-receptor antagonists, NSAIDs or anti-convulsants were not associated with risk of NHL or its major subtypes Citation[158]. However, lifetime use of antibiotics was positively associated with risk of NHL, but it is unclear whether the association was due to use of the antibiotic drugs per se, the infections they were intended to treat or, alternatively, to an underlying susceptibility to infections Citation[158].
Anthropometric measures
The prevalence of obesity has expanded into a global epidemic over recent decades Citation[159], in parallel with the rise in NHL incidence. Several studies have found a significant positive association between obesity and lymphoma incidence Citation[115], Citation[160], but equally many studies have observed no association Citation[161], Citation[162]. Recent results from the Scandinavian case-control study showed no association between usual adult body mass index and risk of NHL overall, but suggested an increased risk of diffuse large B-cell lymphoma among individuals with high body mass index Citation[163]. However, in a prospective cohort study, no association was observed with risk of diffuse lymphomas (n = 137) Citation[164]. Thus, based on available evidence, it appears unlikely that the escalation of overweight and obesity worldwide has contributed meaningfully to the increase in incidence of NHL.
Hair dyes
An excess risk of NHL has been proposed in association with use of hair dyes, especially in women and for dark dye colors, but results are not consistent Citation[165], Citation[166]. In a recent population-based case-control study, an increased risk of NHL was noted only in relation to hair dye use before 1980 Citation[167]. This observation may reflect long duration of use, but it may also relate to the removal of carcinogenic compounds in hair dyes after a warning from the USA Federal Drug Adminstration in 1979 Citation[56].
Other environmental factors
Because pregnancy causes immunologic alterations, it has been suggested that reproductive factors could affect lymphoma incidence. However, no clear picture of any such relationship has emerged Citation[168], Citation[169]. Evidence is also inconclusive with regard to vaccination history Citation[123]. Physical activity could be of importance in lymphomagenesis as exercise is accompanied by transient changes of lymphocyte function. However, neither positive nor negative associations between physical activity and NHL risk has been reported Citation[2]. Exposure to ionizing radiation, either in individuals exposed to therapeutic, diagnostic or occupational radiation or in atomic bomb survivors, does not seem to increase risk of NHL, although it may increase risk of CLL Citation[8], Citation[40]. Acute and chronic psychological stress have suppressive effects on immune function which may affect immune surveillance of developing tumors Citation[170]. A relationship between stress and the development of breast cancer has been indicated Citation[171], but if stressful life events affect the development of malignant lymphomas is not known.
Summary of non-Hodgkin lymphoma etiology
In spite of intense research efforts in recent years, the causes of many cases of NHL remain poorly understood. Established risk factors for NHL overall, or for one or several NHL subtypes, currently include hereditary immunodeficiency disorders, acquired states of strong immunosuppression (HIV/AIDS, organ transplantation), some infectious agents (EBV, HTLV-1, HHV-8, H. pylori), some autoimmune disorders (RA, Sjögren's syndrome, SLE, myositis, Hashimoto's thyroiditis, celiac disease/dermatitis herpetiformis) and a positive family history of hematolymphoproliferative malignancies. Although the list of probable and possible risk factors is clearly growing, evidence is still inconclusive for a several potentially lymphomagenic exposures. A large number of recently conducted or ongoing studies of lymphoma etiology in different countries, as well as international efforts of pooling of results (http://epi.grants.cancer.gov/InterLymph), will hopefully arrive to straighten out some of these uncertainties. Perhaps the most important breakthrough in recent years was the finding that local H. pylori infection is involved in the development of gastric MALT lymphoma, a model that may serve to illustrate the importance of considering biologically plausible risk factors for separate lymphoma entities in current and future research.
A Scandinavian lymphoma etiology initiative
A large case-control study (the SCALE, Scandinavian Lymphoma Etiology, study) was undertaken in Denmark and Sweden between 1999 and 2002 in order to investigate risk factors for malignant lymphomas (previously described in Citation[111]). In brief, individuals aged 18 to 74 years with a first, newly diagnosed malignant lymphoma (NHL, including CLL, or HL according to the WHO classification Citation[1]) were eligible as case patients, and identified through a network of contact physicians in all hospital clinics where malignant lymphomas are diagnosed and treated ( in total 157 departments in both countries). Control subjects were randomly sampled from the entire Danish and Swedish populations (18–74 years) using updated population registers, and were frequency-matched within each country on the expected distribution of NHL cases by sex and age (in 10-year intervals). Review of tumor material took place within the national Lymphoma Registry Organization (LYFO, Citation[172]) in Denmark, and was performed by one of six especially appointed senior hematopathologists or cytologists in Sweden.
Information on potential risk factors for lymphoma was collected through a telephone interview based on a standardized questionnaire, and addressed a number of areas such as medical history, medication and life-style habits. Altogether, close to 7 000 individuals (malignant lymphoma patients and controls) participated in the study (). Most interview participants also gave blood. In Sweden, additional written questionnaires about dietary habits or occupational exposures were administered. The SCALE study constitutes the largest case-control study on etiology of malignant lymphomas hitherto performed internationally. Results from the study have so far been published regarding lymphoma risk and exposure to UV radiation Citation[111], body mass Citation[163], smoking Citation[142], medication Citation[158], autoimmunity Citation[93], diet and alcohol Citation[145], Citation[149] and family history of cancer Citation[32] (see above).
Table I. Number of participants in the SCALE (Scandinavian Lymphoma Etiology) study according to malignant lymphoma status and the WHO classification.
References
- ESHN Jaffe, Stein, H, Vardiman, JW, editors. Pathology and genetics of tumours of hematopoietic and lymphoid tissues. Lyon: IARC Press; 2001.
- Chiu BC, Weisenburger DD. An update of the epidemiology of non-Hodgkin's lymphoma. Clin Lymphoma 2003; 4: 161–8
- A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993;329:987–94.
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000; 403: 503–11
- Cancer IAfRo. Globocan. Cancer Incidence, Mortality and Prevalence Worldwide. In: J Ferlay, Bray, P, Pisani, P, Parkin, DM, editors. Lyon: IARC Press; 2001.
- Cancer Incidence in Sweden 2003. Stockholm: 2005.
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol 2001; 2: 533–43
- Baris D, Zahm SH. Epidemiology of lymphomas. Curr Opin Oncol 2000; 12: 383–94
- Coleman, MP, Esteve, J, Damiecki, P, Arslan, A, Renard, H. Trends in cancer incidence and mortality. IARC Sci Publ 1993;1–806.
- Hakulinen T, Andersen A, Malker B, Pukkala E, Schou G, Tulinius H. Trends in cancer incidence in the Nordic countries. A collaborative study of the five Nordic Cancer Registries. Acta Pathol Microbiol Immunol Scand Suppl 1986; 288: 1–151
- Zheng T, Mayne ST, Boyle P, Holford TR, Liu WL, Flannery J. Epidemiology of non-Hodgkin lymphoma in Connecticut. 1935–1988. Cancer 1992; 70: 840–9
- Devesa SS, Fears T. Non-Hodgkin's lymphoma time trends: United States and international data. Cancer Res 1992; 52: S5432–40
- Registries, AoNC. NORDCAN. Copenhagen: 2004.
- Clarke CA, Glaser SL. Changing incidence of non-Hodgkin lymphomas in the United States. Cancer 2002; 94: 2015–23
- Herrinton LJ. Epidemiology of the Revised European-American Lymphoma Classification subtypes. Epidemiol Rev 1998; 20: 187–203
- Banks PM. Changes in diagnosis of non-Hodgkin's lymphomas over time. Cancer Res 1992; 52: S5453–5
- Hartge P, Devesa SS. Quantification of the impact of known risk factors on time trends in non-Hodgkin's lymphoma incidence. Cancer Res 1992; 52: S5566–9
- Wigzell H. Vårt fantastiska immunförsvar. Liber, Stockholm 1987
- Offit K, Wong G, Filippa DA, Tao Y, Chaganti RS. Cytogenetic analysis of 434 consecutively ascertained specimens of non-Hodgkin's lymphoma: Clinical correlations. Blood 1991; 77: 1508–15
- Ye BH. BCL-6 in the pathogenesis of non-Hodgkin's lymphoma. Cancer Invest 2000; 18: 356–65
- Smith, MT, Skibola, CF, Allan, JM, Morgan, GJ. Causal models of leukaemia and lymphoma. IARC Sci Publ 2004;373–92.
- Filipovich AH, Mathur A, Kamat D, Shapiro RS. Primary immunodeficiencies: Genetic risk factors for lymphoma. Cancer Res 1992; 52: S5465–7
- Aboulafia DM, Pantanowitz L, Dezube BJ. AIDS-related non-Hodgkin lymphoma: Still a problem in the era of HAART. AIDS Read 2004; 14: 605–17
- International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst 2000;92:1823–30.
- Killebrew D, Shiramizu B. Pathogenesis of HIV-associated non-Hodgkin lymphoma. Curr HIV Res 2004; 2: 215–21
- Opelz G, Dohler B. Lymphomas after solid organ transplantation: A collaborative transplant study report. Am J Transplant 2004; 4: 222–30
- Andreone P, Gramenzi A, Lorenzini S, Biselli M, Cursaro C, Pileri S, et al. Posttransplantation lymphoproliferative disorders. Arch Intern Med 2003; 163: 1997–2004
- Zhu K, Levine RS, Gu Y, Brann EA, Hall I, Caplan LS, et al. Non-Hodgkin's lymphoma and family history of malignant tumors in a case-control study (United States). Cancer Causes Control 1998; 9: 77–82
- Paltiel O, Schmit T, Adler B, Rachmilevitz EA, Polliack A, Cohen A, et al. The incidence of lymphoma in first-degree relatives of patients with Hodgkin disease and non-Hodgkin lymphoma: Results and limitations of a registry-linked study. Cancer 2000; 88: 2357–66
- Chiu BC, Weisenburger DD, Zahm SH, Cantor KP, Gapstur SM, Holmes F, et al. Agricultural pesticide use, familial cancer, and risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev 2004; 13: 525–31
- Goldin LR, Pfeiffer RM, Gridley G, Gail MH, Li X, Mellemkjaer L, et al. Familial aggregation of Hodgkin lymphoma and related tumors. Cancer 2004; 100: 1902–8
- Chang ET, Smedby KE, Hjalgrim H, Porwit-MacDonald A, Roos G, Glimelius B, et al. Family history of hematopoietic malignancy and risk of lymphoma. J Natl Cancer Inst 2005; 97: 1466–74
- Goldin LR, Pfeiffer RM, Li X, Hemminki K. Familial risk of lymphoproliferative tumors in families of patients with chronic lymphocytic leukemia: Results from the Swedish Family-Cancer Database. Blood 2004; 104: 1850–4
- Mainou-Fowler T, Dickinson AM, Taylor PR, Mounter P, Jack F, Proctor SJ, et al. Tumour necrosis factor gene polymorphisms in lymphoproliferative disease. Leuk Lymphoma 2000; 38: 547–52
- Vasku JA, Vasku A, Goldbergova M, Vasku V. Heterozygote AG variant of -596 A/G IL-6 gene polymorphism is a marker for cutaneous T-cell lymphoma (CTCL). Clin Immunol 2004; 113: 256–60
- Hishida A, Matsuo K, Tajima K, Ogura M, Kagami Y, Taji H, et al. Polymorphisms of p53 Arg72Pro, p73 G4C14-to-A4T14 at exon 2 and p21 Ser31Arg and the risk of non-Hodgkin's lymphoma in Japanese. Leuk Lymphoma 2004; 45: 957–64
- Hishida A, Matsuo K, Hamajima N, Ito H, Ogura M, Kagami Y, et al. Polymorphism in the hMSH2 gene (gIVS 12-6T– > C) and risk of non-Hodgkin lymphoma in a Japanese population. Cancer Genet Cytogenet 2003; 147: 71–4
- Soucek P, Sarmanova J, Kristensen VN, Apltauerova M, Gut I. Genetic polymorphisms of biotransformation enzymes in patients with Hodgkin's and non-Hodgkin's lymphomas. Int Arch Occup Environ Health 2002; 75(Suppl)S86–92
- Skibola CF, Forrest MS, Coppede F, Agana L, Hubbard A, Smith MT, et al. Polymorphisms and haplotypes in folate-metabolizing genes and risk of non-Hodgkin lymphoma. Blood 2004; 104: 2155–62
- Caporaso N, Marti GE, Goldin L. Perspectives on familial chronic lymphocytic leukemia: Genes and the environment. Semin Hematol 2004; 41: 201–6
- Zhang Y, Lan Q, Rothman N, Zhu Y, Zahm SH, Wang SS, et al. A putative exonic splicing polymorphism in the BCL6 gene and the risk of non-Hodgkin lymphoma. J Natl Cancer Inst 2005; 97: 1616–8
- Niederman JC, Evans AS, Subrahmanyan L, McCollum RW. Prevalence, incidence and persistence of EB virus antibody in young adults. N Engl J Med 1970; 282: 361–5
- Oertel SH, Riess H. Immunosurveillance, immunodeficiency and lymphoproliferations. Recent Results Cancer Res 2002; 159: 1–8
- Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer 2004; 4: 757–68
- Cancer IAfRo. Epstein-Barr Virus and Kaposi's Sarcoma Herpesvirus/Herpesvirus 8. In: IARC Monographs on the evaluation of carcinogenic risks in humans. Lyon: 1997.
- Judde JG, Lacoste V, Briere J, Kassa-Kelembho E, Clyti E, Couppie P, et al. Monoclonality or oligoclonality of human herpesvirus 8 terminal repeat sequences in Kaposi's sarcoma and other diseases. J Natl Cancer Inst 2000; 92: 729–36
- Jeang KT, Giam CZ, Majone F, Aboud M. Life, death and tax: Role of HTLV-I oncoprotein in genetic instability and cellular transformation. J Biol Chem 2004; 279: 31991–4
- Gisbert JP, Garcia-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin's lymphoma: Systematic review and meta-analysis. Gastroenterology 2003; 125: 1723–32
- Negri E, Little D, Boiocchi M, La Vecchia C, Franceschi S. B-cell non-Hodgkin's lymphoma and hepatitis C virus infection: A systematic review. Int J Cancer 2004; 111: 1–8
- Fiorilli M, Mecucci C, Farci P, Casato M. HCV-associated lymphomas. Rev Clin Exp Hematol 2003; 7: 406–23
- De Re V, De Vita S, Marzotto A, Rupolo M, Gloghini A, Pivetta B, et al. Sequence analysis of the immunoglobulin antigen receptor of hepatitis C virus-associated non-Hodgkin lymphomas suggests that the malignant cells are derived from the rheumatoid factor-producing cells that occur mainly in type II cryoglobulinemia. Blood 2000; 96: 3578–84
- Gisbert JP, Garcia-Buey L, Pajares JM, Moreno-Otero R. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther 2005; 21: 653–62
- Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol 2002; 55: 4–13
- Butel JS, Vilchez RA, Jorgensen JL, Kozinetz CA. Association between SV40 and non-Hodgkin's lymphoma. Leuk Lymphoma 2003; 44(Suppl 3)S33–9
- Engels EA, Viscidi RP, Galloway DA, Carter JJ, Cerhan JR, Davis S, et al. Case-control study of simian virus 40 and non-Hodgkin lymphoma in the United States. J Natl Cancer Inst 2004; 96: 1368–74
- Fisher SG, Fisher RI. The epidemiology of non-Hodgkin's lymphoma. Oncogene 2004; 23: 6524–34
- Shivapurkar N, Harada K, Reddy J, Scheuermann RH, Xu Y, McKenna RW, et al. Presence of simian virus 40 DNA sequences in human lymphomas. Lancet 2002; 359: 851–2
- Vilchez RA, Madden CR, Kozinetz CA, Halvorson SJ, White ZS, Jorgensen JL, et al. Association between simian virus 40 and non-Hodgkin lymphoma. Lancet 2002; 359: 817–23
- Nakatsuka S, Liu A, Dong Z, Nomura S, Takakuwa T, Miyazato H, et al. Simian virus 40 sequences in malignant lymphomas in Japan. Cancer Res 2003; 63: 7606–8
- MacKenzie J, Wilson KS, Perry J, Gallagher A, Jarrett RF. Association between simian virus 40 DNA and lymphoma in the United Kingdom. J Natl Cancer Inst 2003; 95: 1001–3
- Montesinos-Rongen M, Besleaga R, Heinsohn S, Siebert R, Kabisch H, Wiestler OD, et al. Absence of simian virus 40 DNA sequences in primary central nervous system lymphoma in HIV-negative patients. Virchows Arch 2004; 444: 436–8
- Brousset P, de Araujo V, Gascoyne RD. Immunohistochemical investigation of SV40 large T antigen in Hodgkin and non-Hodgkin's lymphoma. Int J Cancer 2004; 112: 533–5
- Hernandez-Losa J, Fedele CG, Pozo F, Tenorio A, Fernandez V, Castellvi J, et al. Lack of association of polyomavirus and herpesvirus types 6 and 7 in human lymphomas. Cancer 2005; 103: 293–8
- Schuler F, Dolken SC, Hirt C, Dolken MT, Mentel R, Gurtler LG, et al. No evidence for simian virus 40 DNA sequences in malignant non-Hodgkin lymphomas. Int J Cancer 2006; 118: 498–504
- de Sanjose S, Shah KV, Domingo-Domenech E, Engels EA, Fernandez de Sevilla A, Alvaro T, et al. Lack of serological evidence for an association between simian virus 40 and lymphoma. Int J Cancer 2003; 104: 522–4
- Rollison DE, Page WF, Crawford H, Gridley G, Wacholder S, Martin J, et al. Case-control study of cancer among US Army veterans exposed to simian virus 40-contaminated adenovirus vaccine. Am J Epidemiol 2004; 160: 317–24
- Engels EA, Katki HA, Nielsen NM, Winther JF, Hjalgrim H, Gjerris F, et al. Cancer incidence in Denmark following exposure to poliovirus vaccine contaminated with simian virus 40. J Natl Cancer Inst 2003; 95: 532–9
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991; 338: 1175–6
- Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994; 330: 1267–71
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol 1996; 178: 122–7
- Isaacson PG. Update on MALT lymphomas. Best Pract Res Clin Haematol 2005; 18: 57–68
- Goodlad JR, Davidson MM, Hollowood K, Ling C, MacKenzie C, Christie I, et al. Primary cutaneous B-cell lymphoma and Borrelia burgdorferi infection in patients from the Highlands of Scotland. Am J Surg Pathol 2000; 24: 1279–85
- Wood GS, Kamath NV, Guitart J, Heald P, Kohler S, Smoller BR, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol 2001; 28: 502–7
- Lecuit M, Abachin E, Martin A, Poyart C, Pochart P, Suarez F, et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med 2004; 350: 239–48
- Ferreri AJ, Guidoboni M, Ponzoni M, De Conciliis C, Dell'Oro S, Fleischhauer K, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst 2004; 96: 586–94
- La Vecchia C, Negri E, Franceschi S. Medical history and the risk of non-Hodgkin's lymphomas. Cancer Epidemiol Biomarkers Prev 1992; 1: 533–6
- Tavani A, La Vecchia C, Franceschi S, Serraino D, Carbone A. Medical history and risk of Hodgkin's and non-Hodgkin's lymphomas. Eur J Cancer Prev 2000; 9: 59–64
- Vineis P, Crosignani P, Sacerdote C, Fontana A, Masala G, Miligi L, et al. Haematopoietic cancer and medical history: A multicentre case control study. J Epidemiol Community Health 2000; 54: 431–6
- Askling J, Ekbom A. Risk of non-Hodgkin's lymphoma following tuberculosis. Br J Cancer 2001; 84: 113–5
- Leandro MJ, Isenberg DA. Rheumatic diseases and malignancy–is there an association?. Scand J Rheumatol 2001; 30: 185–8
- Boffetta P, Gridley G, Lindelof B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. J Invest Dermatol 2001; 117: 1531–7
- Gelfand JM, Berlin J, Van Voorhees A, Margolis DJ. Lymphoma rates are low but increased in patients with psoriasis: Results from a population-based cohort study in the United Kingdom. Arch Dermatol 2003; 139: 1425–9
- Mir-Madjlessi SH, Farmer RG, Easley KA, Beck GJ. Colorectal and extracolonic malignancy in ulcerative colitis. Cancer 1986; 58: 1569–74
- Arseneau KO, Stukenborg GJ, Connors AF, Jr, Cominelli F. The incidence of lymphoid and myeloid malignancies among hospitalized Crohn's disease patients. Inflamm Bowel Dis 2001; 7: 106–12
- Askling J, Brandt L, Lapidus A, Karlén P, Björkholm M, Löfberg R, et al. Risk of hematopoietic cancer in patients with inflammatory bowel disease. Gut 2005; 54: 617–22
- Ehrenfeld M, Abu-Shakra M, Buskila D, Shoenfeld Y. The dual association between lymphoma and autoimmunity. Blood Cells Mol Dis 2001; 27: 750–6
- Kauppi M, Pukkala E, Isomaki H. Elevated incidence of hematologic malignancies in patients with Sjogren's syndrome compared with patients with rheumatoid arthritis (Finland). Cancer Causes Control 1997; 8: 201–4
- Pertovaara M, Pukkala E, Laippala P, Miettinen A, Pasternack A. A longitudinal cohort study of Finnish patients with primary Sjogren's syndrome: Clinical, immunological, and epidemiological aspects. Ann Rheum Dis 2001; 60: 467–72
- Ramos-Casals M, Brito-Zeron P, Yague J, Akasbi M, Bautista R, Ruano M, et al. Hypocomplementaemia as an immunological marker of morbidity and mortality in patients with primary Sjogren's syndrome. Rheumatology (Oxford) 2005; 44: 89–94
- Theander, E, Henriksson, G, Ljungberg, O, Mandl, T, Manthorpe, R, Jacobsson, LTH. Lymphoma and other malignancies in primary Sjögren's syndrome: A cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis 2005; Nov 10; [Epub ahead of print].
- Royer B, Cazals-Hatem D, Sibilia J, Agbalika F, Cayuela JM, Soussi T, et al. Lymphomas in patients with Sjogren's syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood 1997; 90: 766–75
- Voulgarelis M, Dafni UG, Isenberg DA, Moutsopoulos HM. Malignant lymphoma in primary Sjogren's syndrome: A multicenter, retrospective, clinical study by the European Concerted Action on Sjogren's Syndrome. Arthritis Rheum 1999; 42: 1765–72
- Smedby, KE, Hjalgrim, H, Askling, J, Chang, ET, Gregersen, H, Porwit-MacDonald, A, et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst 2005; In Press.
- Hakulinen T, Isomaki H, Knekt P. Rheumatoid arthritis and cancer studies based on linking nationwide registries in Finland. Am J Med 1985; 78: 29–32
- Mellemkjaer L, Linet MS, Gridley G, Frisch M, Moller H, Olsen JH. Rheumatoid arthritis and cancer risk. Eur J Cancer 1996; 32A: 1753–7
- Thomas E, Brewster DH, Black RJ, Macfarlane GJ. Risk of malignancy among patients with rheumatic conditions. Int J Cancer 2000; 88: 497–502
- Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: The effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum 2004; 50: 1740–51
- Zhang Y, Holford TR, Leaderer B, Zahm SH, Boyle P, Morton LM, et al. Prior medical conditions and medication use and risk of non-Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control 2004; 15: 419–28
- Franklin, J, Lunt, M, Bunn, D, Symmons, DP, Silman, AJ. Incidence of lymphoma in a large primary-care derived cohort of inflammatory polyarthritis. Ann Rheum Dis 2005, Oct 25; [Epub ahead of print]
- Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM. Tumor necrosis factor antagonist therapy and lymphoma development: Twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum 2002; 46: 3151–8
- Baecklund E, Ekbom A, Sparen P, Feltelius N, Klareskog L. Disease activity and risk of lymphoma in patients with rheumatoid arthritis: Nested case-control study. BMJ 1998; 317: 180–1
- Kamel OW, Holly EA, van de Rijn M, Lele C, Sah A. A population based, case control study of non-Hodgkin's lymphoma in patients with rheumatoid arthritis. J Rheumatol 1999; 26: 1676–80
- Adami J, Frisch M, Yuen J, Glimelius B, Melbye M. Evidence of an association between non-Hodgkin's lymphoma and skin cancer. BMJ 1995; 310: 1491–5
- Frisch M, Hjalgrim H, Olsen JH, Melbye M. Risk for subsequent cancer after diagnosis of basal-cell carcinoma. A population-based, epidemiologic study. Ann Intern Med 1996; 125: 815–21
- Levi F, Randimbison L, Te VC, La Vecchia C. Non-Hodgkin's lymphomas, chronic lymphocytic leukaemias and skin cancers. Br J Cancer 1996; 74: 1847–50
- McKenna DB, Doherty VR, McLaren KM, Hunter JA. Malignant melanoma and lymphoproliferative malignancy: Is there a shared aetiology?. Br J Dermatol 2000; 143: 171–3
- Goggins WB, Finkelstein DM, Tsao H. Evidence for an association between cutaneous melanoma and non-Hodgkin lymphoma. Cancer 2001; 91: 874–80
- Lens MB, Newton-Bishop JA. An association between cutaneous melanoma and non-Hodgkin's lymphoma: pooled analysis of published data with a review. Ann Oncol 2005; 16: 460–5
- Cartwright R, McNally R, Staines A. The increasing incidence of non-Hodgkin's lymphoma (NHL): The possible role of sunlight. Leuk Lymphoma 1994; 14: 387–94
- Hughes AM, Armstrong BK, Vajdic CM, Turner J, Grulich AE, Fritschi L, et al. Sun exposure may protect against non-Hodgkin lymphoma: A case-control study. Int J Cancer 2004; 112: 865–71
- Smedby KE, Hjalgrim H, Melbye M, Torrang A, Rostgaard K, Munksgaard L, et al. Ultraviolet radiation exposure and risk of malignant lymphomas. J Natl Cancer Inst 2005; 97: 199–209
- Norval M. Effects of solar radiation on the human immune system. J Photochem Photobiol B 2001; 63: 28–40
- Holick MF. Vitamin D: A millenium perspective. J Cell Biochem 2003; 88: 296–307
- Bernstein L, Ross RK. Prior medication use and health history as risk factors for non-Hodgkin's lymphoma: Preliminary results from a case-control study in Los Angeles County. Cancer Res 1992; 52: S5510–5
- Holly EA, Lele C, Bracci PM, McGrath MS. Case-control study of non-Hodgkin's lymphoma among women and heterosexual men in the San Francisco Bay Area, California. Am J Epidemiol 1999; 150: 375–89
- McDuffie HH, Pahwa P, McLaughlin JR, Spinelli JJ, Fincham S, Dosman JA, et al. Non-Hodgkin's lymphoma and specific pesticide exposures in men: Cross-Canada study of pesticides and health. Cancer Epidemiol Biomarkers Prev 2001; 10: 1155–63
- Lee WJ, Cantor KP, Berzofsky JA, Zahm SH, Blair A. Non-Hodgkin's lymphoma among asthmatics exposed to pesticides. Int J Cancer 2004; 111: 298–302
- Grulich AE, Vajdic CM, Kaldor JM, Hughes AM, Kricker A, Fritschi L, et al. Birth order, atopy, and risk of non-Hodgkin lymphoma. J Natl Cancer Inst 2005; 97: 587–94
- Hagstromer L, Ye W, Nyren O, Emtestam L. Incidence of cancer among patients with atopic dermatitis. Arch Dermatol 2005; 141: 1123–7
- Lindelof B, Granath F, Tengvall-Linder M, Ekbom A. Allergy and cancer. Allergy 2005; 60: 1116–20
- Holly EA, Lele C. Non-Hodgkin's lymphoma in HIV-positive and HIV-negative homosexual men in the San Francisco Bay Area: Allergies, prior medication use, and sexual practices. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 15: 211–22
- Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol 2004; 113: 395–400
- Melbye M, Trichopoulos D. Non-Hodgkin's Lymphomas. Textbook of Cancer Epidemiology1st ed, HO Adami, D Hunter, D Trichopoulos. Oxford University Press, New York 2002; 535–49
- Pearce, N, McLean, D. Agricultural exposures and non-Hodgkin's lymphoma. Scand J Work Environ Health 2005;31((Suppl 1)):18–25; discussion 15–17.
- Fritschi L, Benke G, Hughes AM, Kricker A, Turner J, Vajdic CM, et al. Occupational exposure to pesticides and risk of non-Hodgkin's lymphoma. Am J Epidemiol 2005; 162: 849–57
- De Roos AJ, Hartge P, Lubin JH, Colt JS, Davis S, Cerhan JR, et al. Persistent organochlorine chemicals in plasma and risk of non-Hodgkin's lymphoma. Cancer Res 2005; 65: 11214–26
- Garabrant DH, Philbert MA. Review of 2,4-dichlorophenoxyacetic acid (2,4-D) epidemiology and toxicology. Crit Rev Toxicol 2002; 32: 233–57
- Nelson NJ. Studies examine whether persistent organic agents may be responsible for rise in lymphoma rates. J Natl Cancer Inst 2005; 97: 1490–1
- Pyatt D. Benzene and hematopoietic malignancies. Clin Occup Environ Med 2004; 4: 529–55
- Wartenberg D, Reyner D, Scott CS. Trichloroethylene and cancer: Epidemiologic evidence. Environ Health Perspect 2000; 108(Suppl 2)161–76
- Raaschou-Nielsen O, Hansen J, McLaughlin JK, Kolstad H, Christensen JM, Tarone RE, et al. Cancer risk among workers at Danish companies using trichloroethylene: A cohort study. Am J Epidemiol 2003; 158: 1182–92
- Cancer I. A. f. R. o. Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. IARC monographs on the evaluation of carcinogenic risks in humans. Lyon France: 1997.
- Cole P, Trichopoulos D, Pastides H, Starr T, Mandel JS. Dioxin and cancer: A ritical review. Regul Toxicol Pharmacol 2003; 38: 378–88
- Zahm SH, Weisenburger DD, Holmes FF, Cantor KP, Blair A. Tobacco and non-Hodgkin's lymphoma: combined analysis of three case-control studies (United States). Cancer Causes Control 1997; 8: 159–66
- Adami J, Nyren O, Bergstrom R, Ekbom A, Engholm G, Englund A, et al. Smoking and the risk of leukemia, lymphoma, and multiple myeloma (Sweden). Cancer Causes Control 1998; 9: 49–56
- Miligi L, Seniori Costantini A, Crosignani P, Fontana A, Masala G, Nanni O, et al. Occupational, environmental, and life-style factors associated with the risk of hematolymphopoietic malignancies in women. Am J Ind Med 1999; 36: 60–9
- Willett EV, Smith AG, Dovey GJ, Morgan GJ, Parker J, Roman E. Tobacco and alcohol consumption and the risk of non-Hodgkin lymphoma. Cancer Causes Control 2004; 15: 771–80
- Freedman DS, Tolbert PE, Coates R, Brann EA, Kjeldsberg CR. Relation of cigarette smoking to non-Hodgkin's lymphoma among middle-aged men. Am J Epidemiol 1998; 148: 833–41
- Stagnaro E, Tumino R, Parodi S, Crosignani P, Fontana A, Masala G, et al. Non-Hodgkin's lymphoma and type of tobacco smoke. Cancer Epidemiol Biomarkers Prev 2004; 13: 431–7
- Talamini, R, Polesel, J, Montella, M, Maso, LD, Crispo, A, Spina, M, et al. Smoking and non-Hodgkin lymphoma: Case-control study in Italy. Int J Cancer 2005. 115: 606–10
- Morton LM, Holford TR, Leaderer B, Boyle P, Zahm SH, Zhang Y, et al. Cigarette smoking and risk of non-Hodgkin lymphoma subtypes among women. Br J Cancer 2003; 89: 2087–92
- Schollkopf C, Smedby KE, Hjalgrim H, Rostgaard K, Gadeberg O, Roos G, et al. Cigarette smoking and risk of non-Hodgkin's lymphoma–a population-based case-control study. Cancer Epidemiol Biomarkers Prev 2005; 14: 1791–6
- Bell DA, Liu Y, Cortopassi GA. Occurrence of bcl-2 oncogene translocation with increased frequency in the peripheral blood of heavy smokers. J Natl Cancer Inst 1995; 87: 223–4
- Schroeder JC, Olshan AF, Baric R, Dent GA, Weinberg CR, Yount B, et al. A case-control study of tobacco use and other non-occupational risk factors for t(14;18) subtypes of non-Hodgkin's lymphoma (United States). Cancer Causes Control 2002; 13: 159–68
- Chang ET, Smedby KE, Zhang SM, Hjalgrim H, Melbye M, Ost A, et al. Alcohol intake and risk of non-Hodgkin lymphoma in men and women. Cancer Causes Control 2004; 15: 1067–76
- Morton LM, Zheng T, Holford TR, Holly EA, Chiu BC, Costantini AS, et al. Alcohol consumption and risk of non-Hodgkin lymphoma: A pooled analysis. Lancet Oncol 2005; 6: 469–76
- Ward MH, Zahm SH, Weisenburger DD, Gridley G, Cantor KP, Saal RC, et al. Dietary factors and non-Hodgkin's lymphoma in Nebraska (United States). Cancer Causes Control 1994; 5: 422–32
- Zheng T, Holford TR, Leaderer B, Zhang Y, Zahm SH, Flynn S, et al. Diet and nutrient intakes and risk of non-Hodgkin's lymphoma in Connecticut women. Am J Epidemiol 2004; 159: 454–66
- Chang ET, Smedby KE, Zhang SM, Hjalgrim H, Melbye M, Ost A, et al. Dietary factors and risk of non-hodgkin lymphoma in men and women. Cancer Epidemiol Biomarkers Prev 2005; 14: 512–20
- Cerhan JR, Wallace RB, Folsom AR, Potter JD, Munger RG, Prineas RJ. Transfusion history and cancer risk in older women. Ann Intern Med 1993; 119: 8–15
- Talamini R, Montella M, Crovatto M, Dal Maso L, Crispo A, Negri E, et al. Non-Hodgkin's lymphoma and hepatitis C virus: A case-control study from northern and southern Italy. Int J Cancer 2004; 110: 380–5
- Zhang Y, Holford TR, Leaderer B, Boyle P, Zahm SH, Owens PH, et al. Blood transfusion and risk of non-Hodgkin's lymphoma in Connecticut women. Am J Epidemiol 2004; 160: 325–30
- Chow EJ, Holly EA. Blood transfusions and non-Hodgkin's lymphoma. Epidemiol Rev 2002; 24: 269–79
- Adami J, Nyren O, Bergstrom R, Ekbom A, McLaughlin JK, Hogman C, et al. Blood transfusion and non-Hodgkin lymphoma: Lack of association. Ann Intern Med 1997; 127: 365–71
- Kato I, Koenig KL, Shore RE, Baptiste MS, Lillquist PP, Frizzera G, et al. Use of anti-inflammatory and non-narcotic analgesic drugs and risk of non-Hodgkin's lymphoma (NHL) (United States). Cancer Causes Control 2002; 13: 965–74
- Beiderbeck AB, Holly EA, Sturkenboom MC, Coebergh JW, Stricker BH, Leufkens HG. Prescription medications associated with a decreased risk of non-Hodgkin's lymphoma. Am J Epidemiol 2003; 157: 510–6
- Beiderbeck AB, Holly EA, Sturkenboom MC, Coebergh JW, Stricker BH, Leufkens HG. No increased risk of non-Hodgkin's lymphoma with steroids, estrogens and psychotropics (Netherlands). Cancer Causes Control 2003; 14: 639–44
- Chang ET, Smedby KE, Hjalgrim H, Schollkopf C, Porwit-MacDonald A, Sundstrom C, et al. Medication use and risk of non-Hodgkin's lymphoma. Am J Epidemiol 2005; 162: 965–74
- James PT. Obesity: The worldwide epidemic. Clin Dermatol 2004; 22: 276–80
- Skibola CF, Holly EA, Forrest MS, Hubbard A, Bracci PM, Skibola DR, et al. Body mass index, leptin and leptin receptor polymorphisms, and non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev 2004; 13: 779–86
- Moller H, Mellemgaard A, Lindvig K, Olsen JH. Obesity and cancer risk: A Danish record-linkage study. Eur J Cancer 1994; 30A: 344–50
- Zhang S, Hunter DJ, Rosner BA, Colditz GA, Fuchs CS, Speizer FE, et al. Dietary fat and protein in relation to risk of non-Hodgkin's lymphoma among women. J Natl Cancer Inst 1999; 91: 1751–8
- Chang ET, Hjalgrim H, Smedby KE, Akerman M, Tani E, Johnsen HE, et al. Body mass index and risk of malignant lymphoma in Scandinavian men and women. J Natl Cancer Inst 2005; 97: 210–8
- Cerhan JR, Janney CA, Vachon CM, Habermann TM, Kay NE, Potter JD, et al. Anthropometric characteristics, physical activity, and risk of non-Hodgkin's lymphoma subtypes and B-cell chronic lymphocytic leukemia: A prospective study. Am J Epidemiol 2002; 156: 527–35
- Correa A, Jackson L, Mohan A, Perry H, Helzlsouer K. Use of hair dyes, hematopoietic neoplasms, and lymphomas: A literature review. II. Lymphomas and multiple myeloma. Cancer Invest 2000; 18: 467–79
- Tavani A, Negri E, Franceschi S, Talamini R, Serraino D, La Vecchia C. Hair dye use and risk of lymphoid neoplasms and soft tissue sarcomas. Int J Cancer 2005; 113: 629–31
- Zhang Y, Holford TR, Leaderer B, Boyle P, Zahm SH, Flynn S, et al. Hair-coloring product use and risk of non-Hodgkin's lymphoma: A population-based case-control study in Connecticut. Am J Epidemiol 2004; 159: 148–54
- Adami HO, Tsaih S, Lambe M, Hsieh C, Adami J, Trichopoulos D, et al. Pregnancy and risk of non-Hodgkin's lymphoma: A prospective study. Int J Cancer 1997; 70: 155–8
- Zhang Y, Holford TR, Leaderer B, Boyle P, Zahm SH, Zhang B, et al. Menstrual and reproductive factors and risk of non-Hodgkin's lymphoma among Connecticut women. Am J Epidemiol 2004; 160: 766–73
- Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol 2004; 5: 617–25
- Bryla CM. The relationship between stress and the development of breast cancer: A literature review. Oncol Nurs Forum 1996; 23: 441–8
- d'Amore FCB, Brincker H, Pedersen NT, Thorling K, Hastrup J, Pedersen MJM, Johansen P, Andersen E. Clinicopathological features and prognostic factors in extranodal non-Hodgkin lymphomas. Danish LYFO Study Group. Eur J Cancer 1991; 27: 1201–08