Abstract
The purpose of this study was to evaluate a single institution's outcome for patients with advanced laryngeal cancer treated with accelerated radiotherapy (RT). Fifty-eight patients with advanced laryngeal cancer (T3/T4N0/N + M0) were treated with curative intent with accelerated RT during the period 1990 − 1998. Patients received radiotherapy alone or with induction chemotherapy. The 5-year local control (LC) and loco-regional control (LRC) probabilities were both 49% for T3 and 75% for T4 tumors. The 5-year disease-free survival probability was 46% and 68% and overall survival probability was 30% and 39% for T3 and T4 tumors respectively. No significant statistical difference in outcome was found, either between T3 and T4 tumors, or between patients who received induction chemotherapy and those who did not. The treatment results for advanced laryngeal cancer at this institution were comparable to those reported in the literature. The results for T3 and T4 were similar. T4 classification alone should not be an exclusion criterion for larynx preservation. Overall survival was poor, partly because of a high incidence of deaths from intercurrent diseases.
In our department treatment for early laryngeal cancer is radical radiotherapy (RT) with surgery saved for salvage. This strategy yields satisfactory results with respect to tumor control, survival and laryngeal function in most patients Citation[1]. For T2 tumors accelerated fractionation of RT has given similar local control (LC) as for conventionally fractionated T1 tumors, suggesting the importance of a short treatment time on RT results even for a low tumor stage Citation[1]-a finding later supported by Garden et al Citation[2].
For advanced T3 and T4 tumors of the larynx both loco-regional control (LRC) and survival have been low in our institution as well as in others, with the treatment strategy of conventionally fractionated RT with surgical salvage Citation[3].
In an attempt to improve the results accelerated RT, as scheduled for T2 tumors, was introduced in our institution in 1990 for advanced stage T3 and T4 tumors of the larynx. This new regimen also included induction chemotherapy for patients with a decent performance status (Karnofsky Index ≥70). The purpose of the induction chemotherapy was two-fold: 1) to reduce the risk of distant metastasis and 2) to shrink the primary tumor.
So far, most data concerning altered RT fractionation schedules for tumors in the head and neck region have dealt with heterogenous patient populations comprising various tumor sites: larynx, oral cavity, oropharynx and hypopharynx. The present study, however, deals with accelerated RT for advanced laryngeal cancer only. All patients have been treated in a single Swedish institution with quality controlled RT. Selection criteria for the choice of treatment have been strict and well described and the patient population has been well defined. The purpose of the present retrospective study was to explore the hypothesis that accelerated RT for advanced laryngeal cancer improves LRC and that induction chemotherapy reduces the incidence of distant metastasis as first site of failure.
Methods and material
Patients
Fifty-eight patients (51 men and 7 women) with advanced laryngeal cancer were treated with curative intent with accelerated RT at the Department of Oncology, Sahlgrenska University Hospital during the period 1990–1998. All patients had tumors classified as T3 or T4, all of which were squamous cell carcinomas. During the same period, 16 additional patients, also diagonsed with locally advanced laryngeal cancer, were not included in this study for the following reasons: 8 patients (1 T3, 7 T4) were treated with primary surgery, 3 patients (all T3) were included in a clinical trial prescribing conventionally fractionated RT, 4 patients (1 T3, 3 T4) were evaluated as being physically or mentally unfit for accelerated fractionation and one patient (T3) was treated with conventionally fractionated RT for reasons not specified in the patient record. The reasons for choosing primary surgery for the eight patients above were either very extensive cartilage involvement, where the chances to obtain LRC with radical RT were considered minimal, or the patient was evaluated as not being able to tolerate RT and/or induction chemotherapy. Of the patients treated with primary surgery, 2 are alive with LRC, 2 died of local recurrences, 2 died of regional recurrences and 2 died of intercurrent diseases. For the patients treated with conventionally fractionated RT, the outcome was as follows: 2 are alive with LRC, 3 died of local recurrences, 1 died of regional recurrence, and, 2 died of intercurrent diseases.
For the patients included in the study, patient and tumor characteristics can be seen in . The distribution of treatment parameters can be seen in .
Table I. Number and distribution of patient parameters
Table II. Distribution of treatment parameters
Patients were referred to our clinic from a number of hospitals in the southwestern region of Sweden and all were discussed at joint tumor conferences including oto-rhino-laryngologists, oncologists, radiologists and histopathologists before allocation to treatment. The diagnostic work-up consisted of clinical examination, evaluation and biopsy under general anesthesia, chest X-ray and a CT (48 patients), MRI (2 patients) or both (8 patients) of the larynx and neck. Tumors were classified and staged at the joint tumor conference according to the 1987 or subsequently updated versions of the UICC staging system Citation[4].
Chemotherapy
Induction chemotherapy consisted of cisplatin 100 mg/m2 day 1 and 5-FU 1000 mg/m2/d by continuous infusion days 1 through 5. The cycle interval was 21 days. Forty-two of the 58 patients were selected for induction chemotherapy. Fifteen (36%) of these patients had T3N0M0 tumors, 22 (52%) had T4N0M0 and 5 (12%) had T4N + M0 tumors (1 N1, 2 N2). Most patients received two cycles of chemotherapy. Four patients who suffered severe complications after their first cycle of chemotherapy received only one cycle. Another two patients received a maximum of three cycles due to long waiting times for the start of RT. Before the start of treatment, all patients went through a physical examination, a hearing examination (audiogram), chest X-ray, ECG and laboratory tests including blood cell count and liver- and kidney function tests. After the completion of two cycles of chemotherapy the tumor response was assessed by physical examination and fiberlaryngoscopy. If there were signs of progression the patient would be referred for laryngectomy.
Sixteen patients (10 T3N0M0, 1 T3N2M0, 3 T4N0M0 and 2 T4N1M0) did not receive induction chemotherapy because of poor performance status, mainly due to high age and/or co-morbidity.
Patients treated with induction chemotherapy had a median age of 63 years compared to 73 years (P = 0.0006) for those who did not receive induction chemotherapy, reflecting treatment selection.
Radiation treatment
All patients were immobilized in a plastic mask excluding the neck and treated on linear accelerators with 4MV, 5MV or 6MV photon beams (). A conformal 3D RT technique mainly with MLC (Multi- Leaf Collimator) was used for all patients. The planning target volume for the full dose treatment (PTV-T) consisted of gross tumor volume (GTV) with a margin of 2 cm, but at least the whole larynx, and positive neck nodes. Bilateral neck nodes including the supraclavicular nodes were included in the adjuvant planning target volume (PTV-N). Gross tumor volume varied greatly, and was between 2 and 112 cm3, depending on neck node involvement. Most patients were treated by two lateral beams often tilted anteriorly 10–20 degrees. These beams often also had a couch rotation of 5–10 degrees. The reason for having a combination of anteriorly directed gantry angles and couch rotation was to avoid the shoulders of the patient. In order to obtain a homogenous dose distribution in the whole planning target volume (PTV), low weighted anterior and posterior fields were added in some cases.
Two different fractionation schedules were used. However, all patients were treated with accelerated fractionation (). Both fractionation schedules had similar toxicity to normal tissues (Biological Equivalent Dose, BED) as calculated from the Linear Quadratic (LQ) formula. Fifty-three patients (25 T3 and 28 T4), of whom 37 had received induction chemotherapy, were treated with split course, hyperfractionated accelerated RT over a total of 4.5 weeks. Patients were treated with fractions of 1.7 Gy, 2 fractions per day with at least 6 hour interval, five days per week. The larynx and positive nodes (PTV-T) were treated up to 64.6 Gy (BEDnormal tissue=115.4 Gy, BEDtumor = 73.2 Gy). Negative nodes, considered as the adjuvant volume (PTV-N), were treated to 40.8Gy (BEDnormal tissue=72.9 Gy, BEDtumor = 50.7 Gy). There was a planned treatment split after 34 Gy in order to reduce acute reactions and late consequential effects.
Five patients (1 T3, 4 T4), who had all received induction chemotherapy, had RT according to a Swedish national multi-center study protocol, evaluating accelerated RT for advanced head and neck cancers, but outside the study. These patients were also treated twice daily, but in a non-split-course regimen with a concomitant boost technique, up to 68 Gy (BEDnormal tissue=120.2 Gy, BEDtumor=70.6 Gy) in 4.5 weeks. In this regimen PTV-N was treated to 46 Gy (BEDnormal tissue=76.7 Gy, BEDtumor=52.2 Gy). The BED values were calculated according to the principles for the LQ model used by Mehta et al Citation[5].
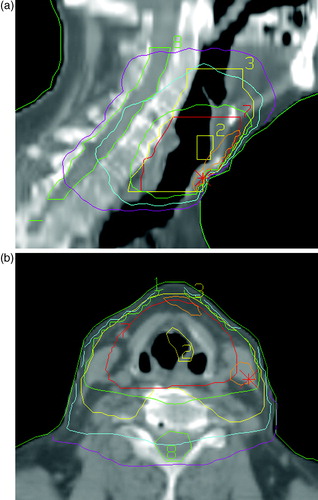
A computer-based 3D RT-planning program was used to calculate the target dose, and the dose was prescribed at the iso-center in the middle of the PTV. The 95% iso-dose of the prescribed dose surrounded the PTV. The PTV-T varied between 77 and 490 cm3 and the PTV-N between 420 and 800 cm3. The lengths of the fields in the cranial caudal direction were in the range of 7–12 cm for the full dose treatment and 12–18 cm for the adjuvant treatment. Target volumes and dose distributions for a typical treatment are illustrated in .
Figure 1. CT slices showing target volumes and dose distributions for a typical radiation treatment in the a) sagittal plane and b) transversal plane. Volumes: Yellow (2) = GTV, Red (7) = PTV (T), Yellow (3) = PTV (N), Green (8) = spinal cord. Isodoses:Orange = 68.0 Gy (105%), Green = 61.4 Gy (95%), Blue = 40.8 Gy (63%), Lilac = 20 Gy (30%) Field sizes:0–40.8 Gy = 14×11 cm 40.8–64.6 Gy = 8×9.5 cm CT = computer tomography, GTV = gross tumor volume, PTV(T) = planning target volume (tumor), PTV(N) = planning target volume (node).
During treatment, all patients were examined weekly by a radiation oncologist who evaluated acute reactions and tumor response.
Follow-up
All patients had their first follow-up examination 4 to 6 weeks after the completion of RT. They were then routinely evaluated under general anesthesia at 3 or 4 months after the completion of RT and if there was any suspicion of local recurrence, a biopsy was taken. Patients were then followed with a clinical examination every 3 months for the first 2 years, every 4 months during the third year and then biannually during the fourth and fifth year. After 5 years follow-up was once a year. A CT scan or MRI of the larynx and neck was performed in most patients at 3 and 6 months after the completion of RT and thereafter only if there was a suspicion of recurrence or other specific indications. Most patients were followed-up at our clinic for the whole study period. However, some patients, especially elderly ones with long traveling distances, were followed at our department during the first and second year, and then at the department of oto-rhino-laryngology at their local hospital. Patients who developed a local recurrence and underwent salvage laryngectomy mostly continued their follow-up at the department of oto-rhino-laryngology Sahlgrenska University Hospital after their laryngectomy.
Three patients were lost to follow-up. One was followed for 50 months. The other two were lost to follow-up directly after and 4.5 months after the completion of treatment respectively. They are all still alive according to the Swedish Population Register. All three were immigrants and we know that at least one of them has returned to his home country.
The mean and median follow-up times were 24.5 months and, 15.3 months, respectively (range, 0.7–117.3). Only 10 patients (17%) could be followed for 5 years or more. Follow-up times were calculated from the last day of treatment to the date of the last follow-up examination or death. Local or regional failure was considered to have occurred when there was either clinical persistence of local and/or regional tumor at the end of treatment or development of recurrence following complete response.
Statistical analysis
The statistical analyses were performed using Stat View, Version 5.0.1 (Abacus Concepts, Inc., CA, USA). The endpoints analyzed were local control (LC), loco-regional control (LRC), disease-free survival (DFS) and overall survival (OS) and were calculated from the first day of treatment by the Kaplan-Meier and actuarial survival methods. OS includes deaths due to any cause. DFS includes deaths due to laryngeal cancer. Patients with local or loco-regional recurrences were treated as failed independently of whether they had salvage surgery or not. P-values for statistical significance were calculated using the log rank test or the unpaired t-test.
Results
–5 show the Kaplan-Meier plots for LRC, DFS and OS probabilities for patients with T3 and T4 tumors respectively. The 5-year survival probabilities are further illustrated in . No statistically significant difference was found between T3 and T4 tumors for any of the parameters analyzed.
Figure 2. Loco-regional control probabilities for patients with T3 (narrow line) and T4 (thick line) tumors.
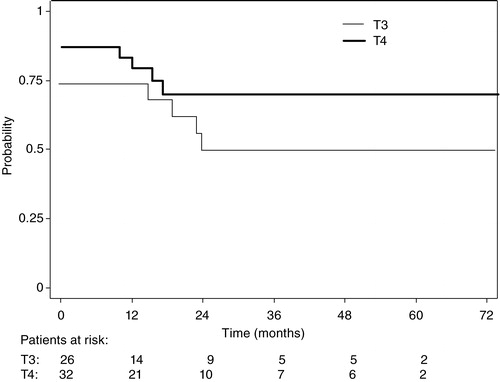
Figure 3. Disease-free survival probabilities for patients with T3 (narrow line) and T4 (thick line) tumors.
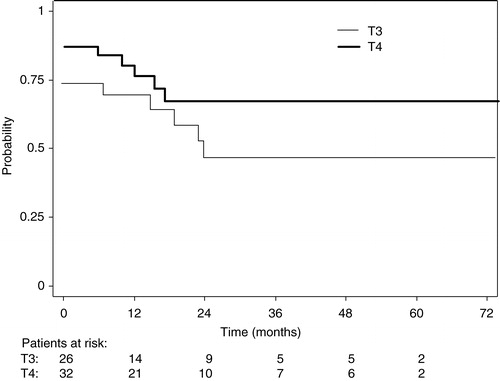
Figure 4. Overall survival probabilities for patients with T3 (narrow line) and T4 (thick line) tumors.
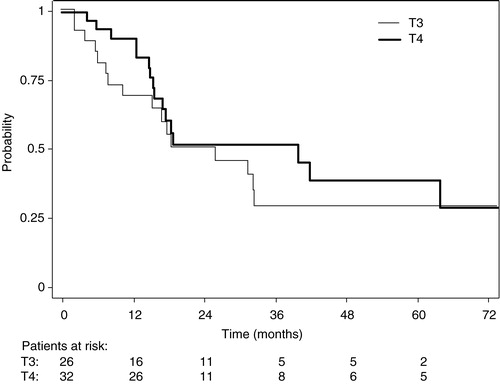
Table III. 5-year survival probabilities for patients with T3 and T4 tumors
The results for patients who received induction chemotherapy versus those who did not can be seen in . No statistically significant difference was found between the two groups for any of the parameters analyzed.
Table IV. 5-year survival probabilities for patients receiving induction chemotherapy versus no induction chemotherapy
Of the 42 patients who received induction chemotherapy 6 (1 T3, 5 T4 = 14%) had a clinical complete remission (CR) and 31 (12 T3, 19 T4 = 74%) had a partial remission (PR) after the completion of chemotherapy. Five patients (2 T3, 3 T4 = 12%) had no response from induction chemotherapy, but went on to have RT as planned. No patient was evaluated as having progressive disease.
After completion of RT 34 patients (11 T3, 23 T4 = 81%) were in CR. Four patients (1 T3, 3 T4) with residual disease after the completion of RT underwent salvage surgery, which was locally successful in two cases (both T4). However, one of them later developed neck disease. Two patients (1 T3, 1 T4) with residual local disease died without an attempt to salvage surgery. One patient (T3) died of pneumonia during his RT, and this was classified as a treatment related death. One patient (T3) was lost to follow up immediately after the completion of RT and could therefore not be evaluated.
Five (2 T3, 3 T4) of the 34 patients who were in CR after RT later developed local recurrences. Three of these patients (1 T3, 2 T4) were treated with salvage surgery, which was successful in 2 cases (both T4). The other two patients died of their local recurrences without any attempt to salvage surgery. Three patients (1 T3, 2 T4 = 7%) developed distant metastases (lung). Two of these also had loco-regional and regional recurrences respectively, while in one patient (T4) distant disease was the first site of failure.
Eleven patients (4 T3, 7 T4 = 26%) who received induction chemotherapy died of intercurrent disease. With information from patients' records and the Swedish national statistical register, as described previously Citation[1], we were able to establish the cause of death in all but one case. The most common cause of death was a second malignancy. One death was considered treatment related, as described above.
From the initial group of 42 patients who received induction chemotherapy 18 patients (5 T3, 13 T4 = 43%) were alive at the time of analysis, out of which 16 (5 T3, 11 T4 = 89%) had a preserved larynx.
Thirteen (7 T3, 6 T4 = 81%) of the 16 patients who did not receive induction chemotherapy had a CR after RT. Two patients (both T3) had persistent local disease and underwent salvage laryngectomy, which was successful in both cases. In one case (T3) response could not be evaluated because the patient died of intercurrent disease shortly after the completion of treatment.
Two patients (1 T3, 1 T4) with complete response after RT developed local recurrences. Both were successfully treated with salvage laryngectomy, but later developed neck disease. One patient (6%) developed neck disease while remaining locally controlled (T4), and one patient (6%) developed distant metastases (lung) as the only site of failure (T3).
Eight patients (5 T3, 3 T4 = 50%) who did not receive induction chemotherapy died of intercurrent disease. The cause of death could be established for seven of these patients. The most common cause of death was a second malignancy. Other causes were pneumonia and cardiovascular disease. The distribution of causes of death for the whole group can be seen in .
Table V. Causes of death
Of the 16 patients who did not receive induction chemotherapy 5 (3 T3, 2 T4 = 31%) were alive at the time of analysis, all with a preserved larynx.
Considering the whole group, twenty-three patients (8 T3, 15 T4) were alive at the time of analysis. Twenty-one of them (8 T3, 13 T4), (91%), had a preserved larynx. Seven patients (17%) experienced complications from their chemotherapy. Three suffered from neutropenic fever and two of them developed sepsis (Common Toxcicity Criteria, CTC grade 3) Citation[6]. They did not receive any further chemotherapy. One patient developed a local infection without neutropenia (CTC grade 2). One patient developed severe trombocytopenia (CTC grade 3), and another patient had cisplatin exchanged with paraplatin because of a moderate decrease in renal function (CTC grade 2). One patient developed a moderate hearing loss caused by cisplatin (CTC grade2).
Seventeen patients (8 T3, 9 T4 = 29%) were noted to have complications or late effects from their treatment. Four of these patients (1 T3, 3 T4), who all experienced persisting pain and laryngeal edema, developed local recurrences within 21 months after completing RT. Two patients, who had both been treated with salvage laryngectomy, postoperatively developed an esophageal stricture and a fistula, respectively. One patient died of pneumonia during his RT, and one died of aspiration pneumonia one month after the completion of RT. The remaining patients had the following complications or late effects: edema requiring temporary tracheostomy (3 patients), edema not requiring tracheostomy (3 patients), temporary pain and swallowing difficulties (1 patient), xerostomia (2 patients).
Discussion
The intention of the treatment policy for laryngeal cancer practiced in this institution is to preserve a functioning larynx whenever possible, without compromising survival. Our LC and LRC results for advanced laryngeal cancer are clearly worse, compared to the LC and LRC for early glottic cancer in our institution also treated with accelerated RT Citation[1]. When compared to the results for advanced laryngeal cancer described in the literature, both for RT and surgery, our results for T3 are in line with those of others and those for T4 are well comparable and perhaps among the better Citation[3], Citation[7–14]. The results for DFS follow well those for LRC. However, OS is poor. Even for the group of patients who received induction chemotherapy, OS was unsatisfactory despite initial good performance status. Nineteen patients dying of intercurrent diseases can explain the poor OS. They comprise as many as 33% of the study population. The most common causes of death were second malignancies (mostly lung), cardiovascular disease and pneumonia, which is almost identical to what we have found for patients with early laryngeal cancer Citation[1]. Despite LC and LRC, patients with laryngeal cancer appear to have a high risk of dying of intercurrent diseases where one common risk factor is likely to be cigarette smoking. This implies that to increase cure of laryngeal cancer, improvement of loco-regional control is essential. However, to increase patient survival, other measures must be taken.
Although not statistically significant, the results for T4 tumors were generally better than for T3 tumors. This might partly be explained by the somewhat higher mean age for the T3 patients and the fact that there were more women among the T4 patients. Also, a larger proportion of the T3 patients had a lower performance status, which was also the reason for more T3 patients not receiving induction chemotherapy (42% compared to 16% of the T4 patients). However, this still contradicts that T4 tumors are less curable when treated with definitive radiotherapy compared to T3 tumors, which is a common opinion in most centers. One explanation could be the positive effect of accelerated RT. Randomized trials on mostly intermediate-stage and advanced head- and- neck cancer, not focusing on laryngeal cancer exclusively, have shown that hyperfractionation and acceleration can improve the results of RT Citation[15], Citation[16]. In the CHART trial for head and neck cancers there was no significant improvement in LRC with CHART, possibly because of the lower total doses. However, a subgroup analysis was performed for the laryngeal tumors, which showed a trend towards a larger benefit of CHART for patients with more advanced disease Citation[17]. This trend found in the CHART-trial supports our results. Even stronger support for the superiority of accelerated RT for loco-regional control of advanced laryngeal cancer was found in the most recent DAHANCA study Citation[18]. It is possible that laryngeal tumors with their particular biological pattern and response to RT, which in many aspects differ from other head and neck tumors, benefit more from accelerated RT.
In this study we did not find any benefit on LRC or survival for those patients who received induction chemotherapy. However, only one patient in the larger induction chemotherapy group developed distant metastasis as the only site of failure. This supports the hypothesis that induction chemotherapy is beneficial for the prevention of distant disease also in laryngeal cancer. The role of induction chemotherapy for head and neck tumors is controversial. One reason for its failure to be beneficial for LC of radical RT could be the resulting increased tumor repopulation after induction chemotherapy. In the present regimen such a repopulation is possibly counteracted by the accelerated RT. A recent meta-analysis by Pignon et al. Citation[19] showed that survival at 5 years was significantly increased when chemotherapy was given concurrently with radiation. The impact of induction chemotherapy could be considered to be too low to be of clinical value. In a subsequent analysis, however, considering studies restricted to induction chemotherapy with cisplatin and 5-FU, 5-year survival was found to be improved by 5%, which is close to the 8% improvement for the concommitant schedules Citation[20]. The toxicity profile shows that induction chemotherapy with cisplatin and 5-FU can be combined with an accelerated RT regimen. The experience of accelerated RT and concurrent chemotherapy is very limited. Considering the more severe acute reaction associated with accelerated RT such a combination should be handled with care. Concommitant chemotherapy and conventionally fractionated RT, however, was superior to induction chemotherapy and RT and to RT alone for advanced laryngeal cancer according to a recent phase III study Citation[21].
The criteria for classifying a tumor as T4, is cartilage invasion, and/or extension of tumor to other tissues beyond the larynx Citation[4]. One can assume that tumors with extension to other tissues beyond the larynx in most cases are large volume tumors, and a large tumor volume has been shown to be a negative prognostic factor for the outcome of RT Citation[22], Citation[23]. Tumors classified as T4 due to cartilage invasion on CT or MRI, however, could represent either a large or a low tumor volume. Some advocate that cartilage invasion by tumor represents a situation in which cure by RT is unlikely Citation[24]. There are today sufficient data to support that this is not the case, as shown in a review by Million Citation[25], and our results could support this. In the present study 20 patients with T4 tumors with cartilage invasion were cured of their laryngeal cancer by RT.
T3 or T4 classification can be difficult to make. Many T4 tumors are underclassified as T3 and T3 tumors are overclassified as T4. CT and MRI are helpful tools in the classification due to their ability to detect cartilage invasion. However, they have their limitations. CT is the most specific of the two modalities (88–94%), but has a lower sensitivity (64–66%) Citation[25], Citation[26] which can result in understaging. MRI has a higher sensitivity (89–95%), but is less specific (76–84%) Citation[26], Citation[27] which leads to overstaging. T4 tumors should therefore not be excluded from RT purely on the basis of the T4 classification. It should be noted that the larynx preservation rate for the patients still alive was high (91%) in this study. A better basis for selecting patients for laryngectomy should be opted for. For such an improvement of selection we need a better understanding of the underlying biology and identification of biological markers to be able to define the group of patients who are likely to fail despite having favorable tumors for cure by RT based on clinical and diagnostic evaluation. However, there will always be a clinical reality where age, performance status and co-morbid illness will select or exclude a patient for a particular treatment modality, and in some cases a laryngectomy may well be the most lenient option.
We thank Dr. Jan Nyman and Dr. Thomas Björk-Eriksson for their help with the patient follow-up, Erik Holmberg for statistical assistance and Åse Blennius for typing the manuscript.
References
- Haugen H, Johansson K-A, Mercke C. Hyperfractionated-accelerated or conventionally fractionated radiotherapy for early glottic cancer. Int J Radiat Oncol Biol Phys 2002; 52: 109–19
- Garden AS, Forster K, Wong P-F, Morrison WH, Schechter NR, Ang KK. Results of radiotherapy for T2N0 glottic carcinoma: does the “2” stand for twice- daily treatment?. Int J Radiat Oncol Biol Phys 2003; 55: 322–8
- Finizia C, Geterud Å, Holmberg E, Lindström J, Lundgren J, Klintberg C, et al. Advanced laryngeal cancer T3-T4 in Sweden: retrospective study 1986–1990. Survival and locoregional control related to treatment. Acta Otolaryngol 1996; 116: 906–12
- Union Internationale Contre le Cancer. TNM Classification of Malignant Tumors. Fourth Fully Revised Edition. Berlin, Heidelberg, New York, London, Paris, Tokyo: Springer-Verlag; 1987. p. 24–26.
- Mehta M, Scrimger R, Mackie R, Paliwal B, Chappell R, Fowler J. A new approach to dose escalation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2001; 49: 23–33
- Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, et al. Common Toxicity Criteria: version 2.0. An improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 2000; 47: 13–47
- Jörgensen K, Godballe C, Hansen O, Bastholt L. Cancer of the larynx. Treatment results after primary radiotherapy with salvage surgery in a series of 1005 patients. Acta Oncol 2002; 41: 69–76
- Nguyen-Tan PF, Le Q-T, Quynh-Thu J-M, Quivey J-M, Singer M, Terris DJ, Goffinet DR, et al. Treatment results and prognostic factors of advanced T3-4 laryngeal carcinoma: the University of California, San Francisco (UCSF) and Stanford University Hospital (SUH) experience. Int J Radiat Oncol Biol Phys 2001; 50: 1172–80
- Harwood AR, Beale FA, Cummings BJ, Hawkins NV, Keane TJ, Rider WD. T3 glottic cancer: an analysis of dose time- volume factors. Int J Radiat Oncol Biol Phys 1980; 6: 675–80
- Parsons JT, Mendenhall WM, Stringer SP, Cassisi NJ. T4 laryngeal carcinoma: radiotherapy alone with surgery reserved for salvage. Int J Radiat Oncol Biol Phys 1998; 40: 549–52
- Karim ABMF, Kralendonk JH, Njo KH, Tierie AH, Hasman A. Radiation therapy for advanced (T3T4N0-N3M0) laryngeal carcinoma: the need for a change of strategy: a radiotherapeutic viewpoint. Int J Radiat Oncol Biol Phys 1987; 13: 1625–33
- Mendenhall WM, Parsons JT, Mancuso AA, Stringer SP, Cassisi NJ. Radiotherapy for squamous cell carcinoma of the supraglottic larynx: an alternative to surgery. Head and Neck 1996; 18: 24–35
- Wang CC, Nakfoor BM, Spiro IJ, Martin SP. Role of accelerated fractionated irradiation for supraglottic carcinoma: assessment of results. Cancer J Sci Am 1997; 3: 88–91
- MacKenzie RG, Franssen E, Balogh JM, Gilbert RW, Birt D, Davidson J. Comparing treatment outcomes of radiotherapy and surgery in locally advanced carcinoma of the larynx: a comparison limited to patients eligible for surgery. Int J Radiat Oncol Biol Phys 2000; 47: 65–71
- Fu KF, Pajak TF, Trotti A, Jones U, Spencer SA, Philips TL, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 2000; 48: 7–16
- Zackrisson B, Mercke C, Strander H, Wennerberg J, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in head and neck cancer. Acta Oncol 2003; 42: 443–61
- Dische S, Saunders M, Barett A, Harvey A, Gibson D, Parmar M. A randomised multicentre trial of CHART versus conventional radiotherapy in head and neck cancer. Radiother Oncol 1997; 44: 123–36
- Overgaard J, Sand Hansen H, Specht L, Overgaard M, Grau C, Andersen E, et al. Five compared to six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6&7 randomised controlled trial. Lancet 2003; 362: 933–40
- Pignon JP, Bourhis J, Domenge C, Designé L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. The Lancet 2000; 355: 949–55
- Monnerat C, Faivre S, Temam S, Bourhis J, Raymond E. End points for new agents in induction chemotherapy for locally advanced head and neck cancers. Ann Oncol 2002; 13: 995–1006
- Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003; 349: 2091–8
- Castelijns JA, Becker M, Hermans R. Impact of cartilage invasion on treatment and prognosis of laryngeal cancer. Eur Radiol 1996; 6: 156–69
- Pameijer FA, Mancuso AA, Mendenhall WM, Parsons JT, Kubilis PS. Can pretreatment computed tomography predict local control in T3 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy?. Int J Radiat Oncol Biol Phys 1997; 37: 1011–21
- Castelijns JA, Golding RP, van Schaik C, Valk J, Snow GB. MR findings of cartilage invasion by laryngeal cancer: value in predicting outcome of radiation therapy. Radiology 1990; 174: 669–73
- Million RR. The myth regarding bone or cartilage involvement by cancer and the likelihood of cure by radiotherapy. Head and Neck 1989; 11: 30–40
- Becker M, Zbären P, Läng H, Stoupis C, Porcellini B, Vock P. Neoplastic invasion of the laryngeal cartilage: comparison of MR imaging and CT with histopathologic correlation. Radiology 1995; 194: 661–9
- Zbären P, Becker M, Läng H. Staging of laryngeal cancer: endoscopy, computed tomography and magnetic resonance versus histopathology. Eur Arch Oto-Rhino- Laryngol Suppl 1997; 50: 1172–80