Abstract
Fifty-four breast carcinomas were studied for the expression of steroid sulfatase (STS) by immunohistochemistry. Correlations between the expression of STS and clinical parameters were determined. Concentrations of serum estrone (E1), estrone sulfate (E1S), estradiol (E2) and estradiol sulfate (E2S) in 12 postmenopausal patients with STS positive tumor were measured by radioimmunoassay. Positive expression of STS was obtained in 72% of tumors. The incidence of STS positive tumor was significantly more frequent in postmenopausal patients (p = 0.01). In our postmenopausal patients, serum E1, E1S, E2, E2S and E2S levels in STS high score group were decreased postoperatively, and those in both STS high and low score group were stabilized after operation. Results from this study suggest STS in breast carcinoma may play an important enzyme of the intratumoral estrogen synthesis in postmenopausal women, and it would be interesting that locally produced STS might be closely related to the control of estrogens environment in breast carcinoma.
Estrogen in breast carcinoma is produced through two main pathways, one from aromatase, which converts androgens to estrogens Citation[1] and the other from steroid sulfatase (STS), which converts estrone sulfate (E1S) into estrone (E1) Citation[2]. In postmenopausal women, these systems are particularly active, and estrogen levels within breast tumors are significantly higher than plasma levels Citation[3]. Much of the E1 formed from androstendione is converted to E1S, which acts as a reservoir for the formation of E1 via STS, and estradiol sulfate (E2S) is also converted by STS to estradiol (E2) Citation[4], Citation[5]. Thus, STS is an important enzyme that converts biological inactivate estrogen sulfate (E1S and E2S) to active free estrogen (E1 and E2).
The success of aromatase inhibitors as a hormone therapy for advanced breast carcinomas has led to a trial in which it is used as adjuvant treatment of breast carcinoma Citation[6]. While treating patients with the new aromatase inhibitors, it was noticed that women with elevated dehydroepiandrosterone sulfate (DHEAS) levels had progressive disease, and women whose DHEA-S levels remained low had stable disease Citation[7]. STS is the enzyme that hydrolyses E1S and DHEAS to their active unsulfoconjugated forms, thereby modulating the growth and survival of estrogen-dependent breast tumors. STS mRNA study showed that the high levels of STS mRNA in breast carcinoma was associated with a significantly shorter relapse free survival rate, but no significant correlation was found between the STS mRNA level and overall survival Citation[8].
Recently, it has been known that STS in breast carcinoma is an important enzyme on the intratumoral estrogen synthesis as same as aromatase. Many studies of STS in breast carcinoma using STS mRNA or the activity of STS were reported, but the immunochemical study of STS in breast carcinoma was a few Citation[9]. The aim of this study was to examine whether expression of STS in breast carcinoma analyzed by immunohistochemistry is associated with clinical parameters, and whether serum estrogens levels in postmenopausal women is changed by removing the tumor with elevated STS expression.
Material and methods
Fifty-four patients who underwent surgical treatment for primary breast carcinoma between 1996 and 1997 in our hospital were included in this study. Informed consent was obtained from all patients. The study was approved from the Ethics Committee in Showa University School of Medicine. Forty carcinomas were from postmenopausal women with a median age of 67 years (range, 50 to 85 years) and 14 were from premenopausal women with a median age of 42.5 years (range, 27 to 49 years). No patient in this study had the distant metastasis from breast carcinoma preoperatively. Also, no patient had received any hormone therapy or chemotherapy prior to surgery. Followed-up information on surviving patients was obtained through March 31, 2003 by our medical note.
All tumors were classified by pathologists according to the WHO scheme for the type of breast tumors. Histologically, there were 46 of invasive ductal carcinoma, two of invasive lobular carcinoma, and six of others. Thirty-seven cases were axially lymph node negative, and 17 cases were lymph node positive. For the size of tumor, 31 were less than 2.0 cm and 23 were 2.0 cm or more.
The surgical specimens were embedded in paraffin, and the serial sections were used for the immunostaining of STS and estrogen receptor (ER). Immunohistochemical staining for STS was performed according to the previous report Citation[10]. Briefly, formalin-fixed, paraffin-embedded tissue was cut into 3 µm sections. The immunohistochemical localization of STS was investigated using the streptavidin-biotin method. Endogenous peroxidase activity was blocked using 3% H2O2 for 20 min at room temperature. After dehydrating and washing with PBS, the immunostaining was performed using polyclonal rabbit anti-human STS antibody Citation[11] diluted in 1:1000. Specificity of the antibody was confirmed by staining a normal human term placenta and STS-deficient placenta diagnosed from Southern blot analysis of the STS gene Citation[12]. MCF-7 breast carcinoma cell, which was obtained from American Type Culture Collection (Rockville, MD), was used for positive control. STS activity of MCF-7 cell was measured by radioimmunoassay (RIA) Citation[13] using 1×106 cells cultured 5 hours, and it was 0.05 pmol/mg. STS mRNA of MCF-7 cell was also detected by reverse transcription polymerase chain reaction (RT-PCR) using the primer in the previous report Citation[10]. Negative control was performed by the STS antibody substitute for a normal rabbit serum and 0.01 ml/L PBS. Immunostaining for ER was carried out using the establised procedure Citation[14].
STS immunohistochemical staining was scored independently and in a blinded manner by two investigators (Y. T. and M. T.). The scoring criteria of the 0, no staining; 1, weak diffuse cytoplasmic staining (may contain stronger intensity in less than 25% of the cancer cells); 2, moderate to strong granular cytoplasmic staining in 25%–50% of cancer cells; 3, over 50% of the tumor cells stained with strong intensity. All specimens with discordant score were reevaluated by the two investigators using a multiheaded microscope, and the consensus score was used for this study. Immunostaining for ER was evaluated as described previously Citation[14], which was positive when 10% or more of cancer cells expressed ER stain, and it was negative when less than 10% of cells.
Eighteen of 54 patients attended the serum steroid hormone assay, such as estrone (E1), estrone sulfate (E1S), estradiol (E2), and estradiol sulfate (E2S), under the informed consent. Serum samples were coded and stored −20°C. Twelve of 18 patients had STS positive tumor in postmenopausal group, and serum estrogens could be measured in samples from those 12 patients before operation, and in samples from eight of 12 patients one day before operation and one month after operation. No adjuvant treatment was performed until one month after operation. Serum E1, E1S, E2 and E2S were analyzed by RIA according to a method described by Gelly et al. Citation[15]. Briefly, aliquots (2 ml) of plasma were pipetted into tubes containing [3H] E1, [3H] E2, [3H] E1S, and [3H] E2S (4000 disintegrations/min of each) at least 30 min before extraction with dichloromethane. The extracts were chromatographed on Sephadex columns to separate E1, E2, E1S, and E2S, and RIA was carried out in duplicate samples.
Correlations between STS immunoreactivity and the clinical factors were determined by the χ2 test. Distant disease free survival (DDFS) was calculated according to the Kaplan-Meier method, and DDFS curves were compared with the log-rank test. Concentration of serum E1, E1S, E2 and E2S were expressed as the mean±SE. Statistical comparisons were made using Mann-Whitney's U test for serum estrogen's levels according to the scoring of STS staining. P < 0.05 was considered statistically significant.
Results
STS expression was observed in the cytoplasma of MCF cells as positive control (). Immunoreactivity of STS was evaluated in 54 invasive breast carcinomas, of which 9.3% (5/54) were score 0 and 19% (10/54) score 1, 20% (11/54) score 2, and 52% (28/54) score3. Positive expression of STS was defined as score 2 or score 3, which was observed in 72% (39/54) of the tumors. Immunoreactivity of STS positive tumors localized exclusively to the cytoplasma of the carcinoma cells, whereas the stroma was either negative or weakly ().
Figure 1. Steroid sulfatase detection in MCF-7 cells by immunohistochemistry with anti-steroid sulfatase antibody. (×400).
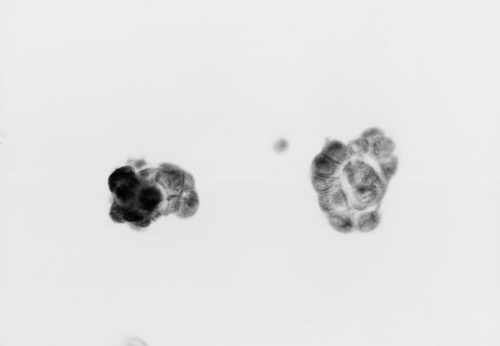
Figure 2. Representative example of steroid sulfatase immunohistochemistry in breast carcinoma. (×200).
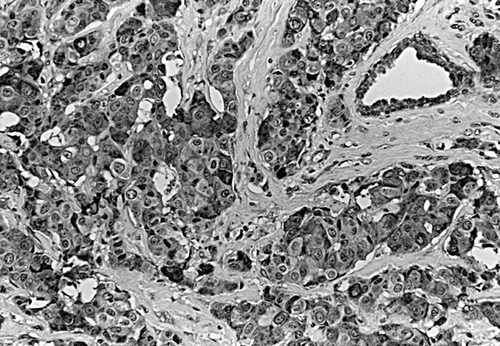
STS positive tumor was significantly more common in tumors with postomenopausal patients as compared with premenopausal patients (p = 0.01). Immunoreactivity of STS was not statistically correlated with the size of tumor, the presence of lymph node metastasis or positive for ER (). Five-year DDFS in the patients with STS positive and negative tumor was 79.5% and 86.7%, respectively. Five-year DDFS was significantly worse in the patients with lymph node metastasis than in those without lymph node metastasis (58.5% vs. 91.9%) (p = 0.003). Similarly, it was significantly worse in the patients with ER negative tumor than in those with ER positive tumor (54.5% vs. 88.4%) (p = 0.005). Regarding tumor size, it was marginally worse in the patients with the size >2cm than in those with the size ≤2cm (69.6% vs. 90.3%) (p = 0.051).
Table I. Association of steroid sulfatase immunoreactivity with clinical parameters.
Twelve serum samples before operation were from the patients with STS positive tumor, in which four samples from STS score 2 and eight samples from STS score 3. In score 2 group, preoperative serum levels of E1, E1S, E2 and E2S were 35.9±5.3 pg/ml (mean±SE), 55.1±7.3, 29.1±4.3, and 75.1±11.0, respectively. In score 3 group, those of E1, E1S, E2 and E2S were 56.0±6.3, 111.7±11.9, 39.9±3.1, and 85.1±11.3, respectively. Serum E1 level in STS score 3 group was higher than in STS score 2 group, although it didn't reach the significant difference (p = 0.06). Serum E1S level in STS score 3 group was significant higher than in STS score 2 group (p = 0.02). No significant difference in either serum E2 or E2S level was found between STS score 3 and score 2 groups ().
Table II. Serum estrogens levels in postmenopausal patients with steroid sulfatase positive tumor.
Serum samples in eight postmenopausal patients with STS positive tumor were used for the examination of change of serum estrogen's levels before and after operation (). There were three paired samples in STS score 2 group, and five paired samples in score 3 group. In STS score 2 group, pre- and post-operative serum E1 level was 32.5±5.8 pg/ml and 36.1±4.6, and E1S level was 51.8±9.2 and 51.6±6.6. E2 level was 26.6±5.0 and 27.0±4.2, and E2S level was 83.4±10.9 and 41.8±5.0. Serum E1, E1S and E2 level examined preoperatively was not significantly different from that examined postoperatively, respectively, but only E2S level was decreased after operation. In the STS score 3 group, pre- and post-operative serum E1 level was 57.9±9.1 and 38.4±3.5, and E1S level was 117.9±15.3 and 54.9±5.0, respectively. E2 level was 37.2±3.0 and 28.3±3.5, and E2S level was 95.7±16.1 and 43.6±3.9, respectively. Concentrations of serum E1, E1S, E2 and E2S of all samples were decreased after operation.
Table III. Change of serum estrogens levels in postmenopausal patients with steroid sulfatase positive tumor before and after operation
Discussion
To our knowledge, the immunocytochemical detection of STS in MCF-7 cells has not been reported, while its activity and mRNA in MCF-7 cells was reported previously Citation[16]. In this study, the immunoreactivity of STS was found in cytoplasma of MCF-7 cells, in which the activity of STS was measured and STS mRNA was detected in our laboratory, and those cells were used for positive control to the immunostaining of STS. STS in breast carcinoma was also stained in cytoplasma of carcinoma cells. Speirs et al. Citation[17] described expression of STS in a series of human breast tumors using RT-PCR, and in epithelial cultures, expression of STS was 77% and expression in stromal cultures was generally reduced. Suzuki et al. Citation[9] reported STS immunoreactivity was detected in carcinoma cells and significantly associated with their mRNA level and enzyme activity, and the positivity of immunostaining was 74.3%. STS positivity of immunochemistry in this study was 72%. These results suggested that the incidence of immunolocalization of STS in carcinoma cells was 70∼75%. Relationship between the tissue levels of STS mRNA and DDFS was reported by Utsumi et al. Citation[8] that patients with breast carcinomas containing high levels of STS mRNA had significantly shorter DDFS as compared with those with low levels of STS mRNA. Yoshimura et al. Citation[18] also reported that patients with high levels of mRNA expression of STS in 155 breast carcinomas had significantly shorter DDFS. Immunohistochemistry has the advantage of the retrospective assessment of STS, and STS can be assessed even in a small number of tumor cells. Suzuki et al. Citation[9] reported STS immunoreactivity in 113 cases of breast carcinoma was significantly associated with an increased risk of recurrence and worsened prognosis. Although our STS positive tumor group showed poorer DDFS than STS negative tumor group, there was no significant difference. This may be caused from the small number of subjects in our study.
The main source of estrogen in premenopausal women is the ovary. In postmenopausal women, the converting enzyme is most important, and the adrenal gland and the extraglandular tissue produce the estrogen's substance, such as androstenedione, DHEAS, E1S, and E2S. STS and aromatase contribute to the high local concentrations of E2 observed within breast tumors of postmenopausal women Citation[19]. STS is responsible for the hydrolysis of E1S to E1 and E2S to E2, and this pathway is of particular importance in breast tumors, as it results in up to ten times freer E1 than the aromatase pathway Citation[20]. In this study, the STS positive tumors in postmenopausal women were significantly more frequent than those in premenopausal women. Our previous study showed the immunoreactivity of aromatase in breast carcinoma was 60%, and in which the postmenopausal group was 76% and the premenopausal group was 24% Citation[21]. These results suggested that STS was an important enzyme, similar to aromatase within the breast carcinoma in postmenopausal women.
It has been well known that E1S level is higher than E2 level both in plasma and tumor of postmenopausal patients, and E1S is 10–20 times higher than E1 in serum concentration, and its half-life is much longer Citation[22]. Moreover, serum E1S levels are higher in postmenopausal women with breast carcinoma than those in normal postmenopausal women Citation[23]. Thus, it is likely that E1S in serum can act as a reservoir for the formation of unconjugated estrogens by the action of STS, and the level of STS activity in human breast tumors is 40–500 times higher than that of aromatase Citation[3]. In our STS score 3 group, serum E1S and E2S level before operation were higher than E2 level, and serum E1S and E2S levels decreased after operation. In STS score 2 group, whereas, serum E1S level before operation was similar to that after operation, and serum E2S level decreased after operation as shown in STS score 3 group. It may suggest when intratumoral STS activity is high, E1 and E2 in breast carcinoma could be produced using plasma E1S and E2S, and when its activity is low, E2S pathway could be active, or DHEAS pathway and aromatase pathway might be superior to E1S pathway. Chetrite et al. Citation[24] reported that the tissue E1, E2 and their sulfates (E1S and E2S), as well as STS and aromatase activities, were evaluated in postmenopausal patients with breast carcinoma, and the values of E2 and E1S, and the activities of STS and aromatase were significantly higher in the tumor tissue than those in area considered as normal. In our postmenopausal patients, serum E1S and E2S levels after operation in STS score 3 group were similar to those in STS score 2 group, and serum E1 and E2 levels after operation were almost same in both groups. This change of serum estrogens may result from removing the tumor with STS activity, and the change of serum estrogens after operation might suggest that the intratumoral estrogen synthesis play an important role in the postmenopausal women with breast carcinoma, and STS in tumors is one of key enzymes as aromatase.
In conclusion, elevated expression of STS by immunohistochemistry was observed in 72% of breast carcinoma, and tumors with positive expression of STS in postmenopausal women were significantly more common than those in premenopausal women. Recently, new sulfatase inhibitor has been developed Citation[25]. The levels of serum steroid hormone such as E1, E2 and E1S, E2S had become stable by one month after operation in the present study, suggesting that those stabilized levels may be suitable when treated with sulfatase inhibitor, although our subjects were small number of Japanese patients. Locally produced STS may be, therefore, closely related to the control of estrogens environment in breast carcinoma, and the effect of the sulfatase inhibitors against breast carcinoma as an endocrine therapy may be expected for the future.
References
- Liton A, Santner S, Santen RJ, et al. Aromatase activity in primary and metastatic human breast cancer. Cancer 1987; 59: 779–82
- Pasqualini JR, Chetrite GS. Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer. J Steroid Biochem Mol Biol 2005; 93: 221–36
- Pasqualini JR, Chetrite G, Blacker C, et al. Concentrations of estrone, estradiol and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and post-menopausal breast cancer patients. J Clin Endocrinol Metab 1996; 81: 1460–4
- Wilking K, Carlstrom K, Gustafsson SA, et al. Oestrogen receptors and metabolism of oestrone sulfate in human mammary carcinoma. Eur J Cancer 1980; 16: 1339–44
- Vignon F, Tergui M, Westley B, et al. Effect of plasma estrogen sulfates in mammary cancer cells. Endocrinology 1980; 106: 1079–86
- Howell A, Cuzick J, Baum M, et al. Results of ATAC (‘Arimidex’, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005; 365: 60–2
- Morris KT, Toth-Fejel S, Schmidt J, Fletcher WS, Pommier RF. High dehydroepiandrosterone-sulfate predicts breast cancer progression during new aromatase inhibitor therapy and stimulates breast cancer cell growth in tissue culture: A renewed role for adrenalectomy. Surgery 2001; 130: 947–53
- Utsumi T, Yoshimura N, Takeuchi S, et al. Steroid sulfatase expression is an independent predictor of recurrence in human breast cancer. Cancer Res 1999; 59: 377–81
- Suzuki T, Nakata T, Miki Y, et al. Estrogen sulfotransferase and steroid sulfatase in human breast carcinoma. Cancer Res 2003; 63: 2762–70
- Yanaihara Y, Yanaihara T, Toma Y, et al. Localization and expression of steroid sulfatase in human fallopian tubes. Steroid 2001; 66: 87–91
- Osawa Y, Higashiyama T, Yarborough C, Shimizu Y, Yanaihara T.. Purification of human estrogen sulfatase and monoclonal antibody preparation. 81st Annual Meeting of The Endocrine Society 1999; Abstr.
- Suzuki T, Hirato K, Yanaihara T, et al. Purification and properties of steroid sulfatase from human placenta. Endocrinol Jpn 1992; 39: 93–101
- Evans TR, Rowiands MG, Law M, Coombes RC. Intratumoral oestrone sulphatase activity as a prognostic marker in human breast carcinoma. Br J Cancer 1994; 69: 555–61
- Kurosumi M. Significance of immunohistochemical assessment of steroid hormone receptor status for breast cancer patients. Breast Cancer 2003; 10: 97–104
- Gelly C, Sumida C, Gulino A, Pasqualini JR. Concentration of estradiol and estrone in plasma, uterus and other tissues of fetal guinea-pigs: Their relationship to uptake and specific binding of [3H]-estradiol. J Endocr 1981; 89: 71–7
- Pasqualini JR, Gelly C, Lecerf F. Estrogen sulfates: Biological and ultrastructural responses and metabolism in MCF-7 human breast cancer cells. Breast Cancer Res Treat 1986; 8: 233–40
- Speirs V, Walton DS, Hall M-C, Atkin SL. In vivo and in vitro expression of steroid-converting enzymes in human breast tumors; associations with interleukin-6. Br J Cancer 1999; 81: 690–5
- Yoshimura N, Harada N, Bukholm, et al. Intratumoral mRNA expression of genes from the oestradiol metabolic pathway and clinical and histopathological parameters of breast cancer. Breast Cancer Res 2004; 6: 46–55
- Reed MJ, Purohit A. Breast cancer and the role of cytokines in regulating estrogen synthesis: An emerging hypothesis. Endo Rev 1997; 18: 701–5
- Santner SJ, Feil PD, Santen RJ. In situ estrogen production via the estrone sulfatase pathway in breast tumors: Relative importance versus the aromatase pathway. J Clin Endo Metab 1984; 59: 29–33
- Tsunoda Y, Kamiya K, Tsunoda A, et al. Immunohistochemical detection of aromatase in breast carcinomas. Showa Univ J Med Sci 2000; 12: 247–52
- Ruder HJ, Loriaux L, Lipsett MB. Estrone sulfate: Production rate and metabolism in man. J Clin Invest 1972; 51: 1020–33
- Prost O, Turrel M, Dahan N, et al. Estrone and dehydroepiandrosterone sulfatase activities and plasma estrone sulfate levels in human breast carcinoma. Cancer Res 1984; 44: 661–4
- Chetrite GS, Cortes-Prieto J, Philippe JC, Wright F, Pasqualini JR. Comparison of estrogen concentrations, estrone sulfatase and aromatase activities in normal, and in cancerous, human breast tissues. J Steroid Biochem Molec Biol 2000; 72: 23–7
- Lloyd MD, Thiyagarajan N, Ho YT, et al. First crystal structures of human carbonic anhydrase II in complex with dual aromatase-steroid sulfatase inhibitors. Biochemistry 2005; 44: 6858–66