Abstract
Radioiodine treatment for thyroid disease has been given for half a decade in Sweden. The most common indication for treatment is hyperthyroidism, when iodine uptake is high. The situation in which radioiodine treatment is used in thyroid cancer is less favourable and measures therefore have to be taken to optimize the treatment. Treatment should be performed early in the course of the disease to achieve the highest possible differentiation. Before treatment the iodine and goitrogen intake should be kept low. Stimulation of the thyrocytes by thyroid-stimulating hormone (TSH) should be high. It is conventionally achieved by thyroid hormone withdrawal rendering the patient hypothyroid, or by the recently available recombinant human TSH (rhTSH) which can be recommended for ablation of the thyroid remnant after thyroidectomy and for treatment of metastases in fragile patients unable to undergo hypothyroidism. Finally, stunning – the negative effect of a prior test dose from radioactive iodine – should be avoided.
The Chernobyl experience has shown without doubt that exposure to radioactive iodine isotopes in young persons can induce differentiated thyroid cancer, with the number of diagnosed children now amounting to about 4 000. It may therefore seem paradoxical that radioiodine is used also to treat the same cancer. This paper addresses the present routines for and problems concerning treatment of highly differentiated thyroid cancer with iodine 131 (131I).
Treatment with radioiodine for thyroid cancer is administered in two situations: firstly, in ablation of residual normal thyroid tissue after “total” thyroidectomy in order to facilitate follow-up with serum measurements of the thyroid-specific glycoprotein thyroglobulin and at the same time provide an adjuvant therapy, thus treating microscopic foci of cancer. This treatment also allows for post-therapy gamma camera evaluation of the patient, which may reveal distant metastases. Secondly, radioiodine treatment is given to patients with known residual tumour or metastases from thyroid cancer.
In ablation after total thyroidectomy an absorbed dose of about 300 Gy to the remnant is normally aimed at Citation[1]. This is supposedly achieved by giving the patient 1 100–4 000 MBq 131I. In many centres today this is administered without prior diagnostic scanning. About three days post-therapy a whole-body scintigraphy is performed and the uptake in the remnant can be calculated. With surgery today aimed at removing as much of the thyroid tissue as possible without serious complications, this often amounts to <1% of the given activity.
The indication for postsurgical ablation therapy with radioiodine has been much debated. It has been argued that the benefit to patients with good prognosis is small and the risk of inducing another cancer though low is not negligible Citation[2]. A consensus report from a group of European experts in the field has recently been published Citation[3]. In this report thyroid cancer patients have been suggested to be divisible into three groups: very-low-risk patients, low-risk patients and high-risk patients. There is no indication for ablation in the very-low-risk group while ablation with about 3 700 MBq is recommended in the high-risk group. For the low-risk group, presence of certain parameters is a probable indication. The amount of activity in this group, however, may be in the lower range, i.e. 1 100–2 000 MBq.
In the treatment of known residual tumour or metastases a somewhat higher amount of activity is often used to achieve an absorbed dose to the cancer cells of >70 Gy, which is considered important if a therapeutic effect is to be achieved. The absorbed dose is dependent on the tumour mass and the uptake of 131I, and also, on the residence time of the radionuclide in the tissue. Activities of 4 000–8 000 MBq are often used to reach as high an absorbed dose as possible. The treatment is often repeated.
It is believed that thyroglobulin is produced in most differentiated thyroid carcinoma cells while iodine uptake is only present in 2/3 of the cases. The iodine uptake diminishes with higher age and progression of disease. Measurement of thyroglobulin has therefore become a very crucial means of follow-up in patients and the test has been shown to have better sensitivity than do diagnostic scans.
In the intact thyroid follicle the radioactive iodine will eventually be bound to thyroglobulin molecules in the follicle lumen and these molecules will then be the main source of radiation () Citation[4], Citation[5]. The iodine uptake in the thyroid follicle is dependent on several metabolic steps. Most important is the iodide uptake by the sodium iodide symporter (NIS) at the basal cell membrane of the thyrocytes, but transporters at the apical membrane, such as pendrin and the apical iodide transporter (AIT), and the oxidation and iodination of thyroglobulin by the enzyme thyroperoxidase (TPO) at the border of the follicle lumen are also crucial parameters in the process.
Figure 1. The thyroid follicle cell with iodinated thyroglobulin molecules (Electron microscopy: G Berg). With kind permission from Nycomed AB and authors.
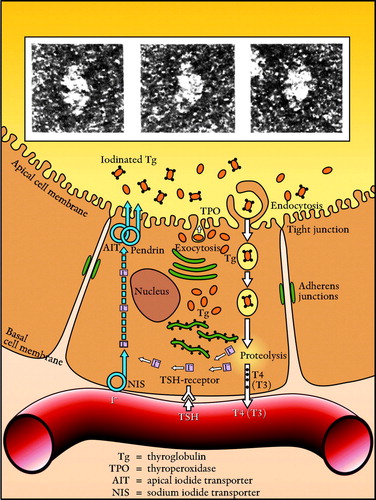
The character and function of the NIS are well described in a review by Dohan et al. Citation[6]. In order to carry iodide through the basal membrane the NIS has to be integrated in the membrane. Anions other than iodide can also be transported by the NIS, such as thiocyanate (SCN-) and perchlorate, and the presence of such anions will therefore compromise the transport.
In order to achieve as high an absorbed dose as possible to the tumour the treatment should be optimized. The most important factors with regard to optimizing treatment will be addressed in the following paragraphs ().
I. The importance of differentiation
High differentiation is important for the NIS function of the thyrocytes. Also, it is inconceivable that intact follicle formation is a prerequisite for a sufficient resident time of the radioiodine. It is therefore important to give the treatment as early as possible in the development of the disease.
One way of increasing the differentiation is to treat the patient with retinoic acid for about five weeks before radioiodine treatment. This treatment is interesting in theory but the results from centres that have tried it are very divergent and not always convincing Citation[7]. Consequently retinoic acid treatment has not become a routine treatment for non-iodine accumulating thyroid cancer. Finding other ways of increasing the differentiation is currently an interesting topic of investigation.
II. Iodine in the thyroid and the importance of low iodine intake
The iodine content in the normal benign thyroid is in the range of 2–20 mg, with an iodine concentration of 0.1–1.0 mg/ml. Knowledge about the iodine content in malignant tissue is scarce, however. We have previously tried to address this issue by studying tissue samples from patients with thyroid cancers using X-ray fluorescence (XRF) analysis Citation[8]. Preliminary results show that the iodine content in the malignant part of the thyroid can be below the detection limit of the method compared with levels of 0.3 mg/ml in the non-malignant part of the thyroid (Hansson et al., communicated at the 13th International Thyroid Conference in Buenos Aires, Argentina, in 2005). This was also demonstrated by using secondary ion mass spectrometry (SIMS), a method allowing detection of iodine in microscopic sections.
Possible mechanisms leading to low iodine content in thyroid cancer have been demonstrated elsewhere using immune staining technique Citation[9]. In that study, immunostaining of thyroid tissue from normal and autonomous as well as follicular and papillary thyroid cancer was performed. It was found that while iodinated thyroglobulin was present in normal and autonomous thyroid tissue it was absent in the cancer tissue studied. In the papillary carcinoma, staining for the NIS was low while in the follicular cancer staining for pendrin was low.
The rationale for having a low iodine load before treatment is that this keeps the dilution with cold iodine as low as possible. It is most important to avoid overload by iodine-containing contrast agents. Iodine contrast does not only give high momentary dilution, but it also resides in several body compartments for a long time Citation[10]. Furthermore, there may be a risk of inducing a destructive thyroiditis in the thyrocytes, destroying the follicle structure and thus inhibiting iodine uptake for a considerable time.
Iodine uptake is dependent on the normal iodine uptake in the population. We have recently shown that the mean 24 hour iodine uptake in a population of 60–65-year olds in western Sweden is 21% Citation[11], which is about half the value measured by Larsson in Sweden in the 1950s Citation[12]. A 24 hour uptake of 21% indicates that the iodine intake in Sweden today may be as high as 300 µg/day compared with about 100 µg/day in the 1950s. This has to be considered and supports the use of a low-iodine diet (LID) in preparation for radioiodine treatment.
With this in mind we tested the effect of a strict LID on subsequent iodine uptake in radioiodine remnant ablation. Unexpectedly, an improvement in uptake using LID compared with a normal diet was not seen. However, a significant improvement in iodine uptake was found when we used a diet which was also low in goitrogens (LILGD). Consequently flavonides, SCN- and glucosinates were excluded (Erik Aaro et al., communicated at the 13th International Thyroid Conference in Buenos Aires, Argentina, in 2005). We therefore recommend a diet low in iodine as well as in goitrogens in preparing the patient for radioiodine treatment.
III. Stimulation with thyroid-stimulating hormone
The function of the NIS is of utmost importance in achieving optimized radioiodine uptake. It has been shown that the NIS is dependent on TSH stimulation with regard to both synthesis (NIS messenger ribonucleic acid (mRNA) and NIS protein) and targeting of the NIS to the cell membrane.
Thyroid-stimulating hormone stimulation can be achieved in two ways, namely by endogenous stimulation by the pituitary after temporary termination of thyroid substitution in thyroidectomized patients, or by exogenous stimulation with recombinant TSH in order to prepare patients for ablative therapy. For a long time, endogenous stimulation was the only available method. With this stimulation the patient will have to develop hypothyroidism with a TSH level >30 mµ/l. A common procedure is to let the patient have the more short-lived hormone triiodothyronine instead of thyroxine for four weeks and then go for two weeks without any thyroid hormone.
Exogenous stimulation is given by administering rhTSH (Thyrogen®) as intramuscular injections of 0.9 mg 48 and 24 hours before radioiodine administration. Using rhTSH and keeping the patient euthyroid has proved to have several beneficial effects. For instance, the patient does not need long sick leave. Also, in the case of residual tumour the tissue is not stimulated for a long period and the residence time of the radioactivity in the body is shorter, thus giving less unwanted radiation to the body Citation[13].
IV. Stunning
The term “stunning” means that the activity administered in order to perform a diagnostic scan gives a dose to the target tissue that will diminish the subsequent uptake of the activity administered for therapy. The first time this phenomenon was mentioned was in 1951 by Rawson et al. Citation[14] without any effect on the routines in nuclear medicine practice. At our Department we first experienced stunning in 1989 when a 12-year-old girl was treated for a large papillary thyroid cancer with massive pulmonary metastasing. After total thyroidectomy the girl was referred to us for radioiodine treatment. As we were concerned not to give too high a dose to the lung we performed a dose calculation by giving the patient 100 MBq 131I as a test dose. Four days after administration 15% of the test activity was found in the lungs. Based on this we calculated that a safe activity would be 3 500 MBq. To our great surprise only 0.7% of this activity was found in the lungs after nine days. We realized that either the uptake was much lower than expected or the effective half-life was much shorter. We also realized that the dose to the metastases regrettably was <10% of the intended dose. In a search of the literature we found Jeevenram et al.'s work from 1986 clearly describing the phenomenon of stunning Citation[15]. After this incident we became very cautious, using low activities of 131I or, alternatively, using the gamma emitter 123I or simply avoiding diagnostic scans.
The uncertainty in the literature concerning stunning eventually led us to approach the Institution of Anatomy and Cell Biology in Göteborg to propose an in vitro experiment using their double-compartment model Citation[16]. We have now shown in vitro that a stunning effect at low radioactive doses indeed exists. In our material, doses of 2.7–81 Gy induced an iodide transport defect in the thyrocytes of 47–94% Citation[17].
Recently a very convincing clinical study by Lassman et al. was published Citation[18] aimed to compare the uptake after withdrawal with the uptake after rhTH. A test activity of 74 MBq was given twice consecutively in the same patient before the therapeutic activity. In the experiment a meticulous dose calculation was performed. The authors found a decrease in uptake of about 44% after the second test activity and, importantly, they also found about 25% lower residence time. The result was independent of whether the rhTSH was given first or as a second step in the experiment. A stunning effect was therefore indeed present, decreasing both initial uptake and resident time.
A review of the stunning effect was recently published indicating that clinically significant stunning may be expected after an absorbed dose as low as 4 Gy Citation[19]. Stunning is now considered to function as a partial ablation, the mechanisms behind which are likely to be multifactorial. For instance, cells not undergoing cell death may show diminished NIS activity leading to a lower iodine uptake; also, a defect in follicle integrity may explain the shorter residence time.
Conclusions
To optimize radioiodine treatment in malignant thyroid cancer the treatment should be performed as soon as possible in the disease in order to benefit from high differentiation. The iodine and goitrogen intake should be kept low before treatment. The TSH level should be high and rhTSH can be highly recommended as preparation for ablation treatment and treatment of metastases in fragile patients unable to undergo hypothyroidism. Finally, stunning should be avoided.
References
- Maxon HR, Smith HS. Radioiodine-131 in the diagnosis and treatment of metastatic well differentiated thyroid cancer. Endocrinol Metab Clin North Am 1990; 19: 685–718
- Rubino C, De Vathaire F, Dottorini ME, Hall P, Schvartz C, Couette JE, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer 2003; 89(9)1638–44
- Pacini F, Schlumberger M, Harmer C, Berg G, Cohen O, Duntas L, et al. Post-surgical use of radioiodine 131I in patients with papillary and follicular thyroid cancer and the issue of remnant ablation: A consensus report. Eur J Endocrinol 2005; 153: 651–9
- Nyström E, Berg G, Jansson S, Lindstedt G, Törring O, Valdemarsson S. Thyreotoxicos hos vuxna. Nyström E, Nycomed AB editors. StockholmSweden; 1999.
- Berg G. The structure of human thyroglobulin. J Ultrastruct Res 1975; 53: 113–8
- Dohan O, De la Veija A, Paroder V, Riedel C, Artani M, Reed Mia, et al. The sodium/iodide symporter (NIS): Characterization, regulation, and medical significance. Endocr Rev 2003; 24(1)48–77
- Coelho SM, Corbo R, Buesco A, Carvalho DP, Vaisman M. Retinoic acid in patients with radioiodine non-responsive thyroid carcinoma. J Endocrinol Invest 2004; 27(4)334–9
- Hansson M, Berg G, Larsson A, Nyström E, Isaksson M. X-ray fluorescence analysis for determination of iodine concentration in the thyroid. A methodological study. Int J Body Composition Res 2004; 2: 155–63
- Gérard A-C, Daumerie C, Mestdagh C, Gohy S, De Burbure S, Costagliola S, et al. Correlation between the loss of thyroglobulin iodination and the expression of thyroid-specific proteins involved in iodine metabolism in thyroid carcinomas. J Clin Endocrinol & Metab 2003; 88: 4977–83
- Van der Molen A, Thomsen HS, Morcos SK, Members of the Contrast Media Safety Committee of the European Society of Urogenital Radiology (ESUR). Effect of iodinated contrast media on thyroid function in adults. Eur Radiol 2004; 14: 902–7
- Milakovic M, Berg G, Eggertsen R, Nyström E. Effect of lifelong iodine supplementation on thyroid 131-I uptake: A decrease in uptake in euthyroid but not hyperthyroid individuals compared to observations 50 years ago. Eur J Clin Nutr 2006; 60: 210–213
- Larsson LG. Studies on radioiodine treatment of thyrotoxicosis with special reference to the behaviour of the radioiodine tracer tests. Acta Radiol 1955; 126: 1–164
- Luster M, Lippi F, Jarzab B, Perros P, Lassmann M, Reiners C, Pacini F. rhTSH-aided radioiodine ablation and treatment of differentiated thyroid carcinoma: A comprehensive review. Endocr Rel Cancer 2005; 12: 49–64
- Rawson RW, Rall JE, Peacook W. Limitations and indications in the treatment of cancer of the thyroid with radioactive iodide. J Clin Endocrinol Metab 1951; 11: 1128–31
- Jeevanram RK, Shah DH, Sharma SM, Ganatra RD. Influence of initial large dose on subsequent uptake of therapeutic radioiodine in thyroid cancer patients. Nucl Med Biol 1986; 13: 277–9
- Nilsson M, Björkman U, Ekholm R, Ericson LE. Iodide transport in primary cultured thyroid follicle cells: Evidence of a TSH-regulated channel mediating iodide efflux selectively across the apical domain of the plasma membrane. Eur J Cell Biol 1990; 52: 270–81
- Postgård P, Himmelman J, Lindencrona U, Bhogal N, Wiberg D, Berg G, et al. Stunning of iodide transport by 131I irradiation in cultured thyroid epithelial cells. J Nucl Med 2002; 43: 828–34
- Lassmann M, Luster M, Hanscheid H, Reiners C. Impact of 131I diagnostic activities on the biokinetics of thyroid remnants. J Nucl Med 2004; 45: 619–25
- Medvedec M. Thyroid stunning in vivo and in vitro. Nucl Med Comm 2005; 26: 731–5