Abstract
p16INK4a, laminin-5γ2 chain, and PCNA were investigated to compare the expression levels in relation to histological diagnosis and time for progression. The material consisted of 37 normal cervical tissues, 35 with different grades of CIN, and 11 invasive cervical cancers. Our results showed a reduction of basement membrane staining for laminin-5γ2 chain from 78.4% in normal squamous epithelium to 27.8% in CIN3 (p < 0.001). The intracytoplasmic staining for laminin-5γ2 chain increases with severity of lesion. The same trend was observed with p16INK4a and PCNA expression (p < 0.001). Co-expression of p16INK4a and PCNA was seen in 85.7% of samples. Cases that were laminin-5γ2 chain BM − /p16INK4a+/PCNA+ have the shortest interval time (average: 46.8±36.3 months) for progression, while cases with laminin-5γ2 chain BM + /p16INK4a−/PCNAPCNA− have the longest time interval (average: 110.2±52.7 months) (p < 0.05). Thus co-expression of p16INK4a, laminin-5γ2 chain and PCNA may be valuable for the prediction progression of cervical neoplasia.
Epidemiological studies as well as experimental studies indicated that infection with the human papillomavirus (HPV) is the most important etiological factor in the development of cervical cancer Citation[1], Citation[2]. A series of different types of HPV has been associated with diseases of the genital tract mucosa. Of these, HPV 16, 18, 45, 31 and 33 are the most prevalent among cervical carcinomas Citation[3], Citation[4]. HPV DNA is present in about 95% of squamous cell carcinomas Citation[4], while in adenocarcinomas of the cervix, it has been detected in only about 70% to 90% Citation[5], Citation[6]. Due to population-based Pap smear screening, the incidence of squamous cell carcinoma has decreased remarkably in most developed and in some developing countries, while during the same period, there has been a relative increase in the incidence of adenocarcinoma among women Citation[7–9]. Several studies have reported this escalation, especially in young women Citation[10]. Cytological screening is apparently not effective in preventing the development of adenocarcinomas Citation[8]. The precancerous stages of invasive cervical carcinoma are defined as different grades of dysplasia. It is suggested that approximately 12% of all carcinomas in-situ, when left untreated, evolved into invasive cancer after at least 13 years. The natural history of cervical intraepithelial neoplasia (CIN) is extremely variable. If left untreated, CIN may regress to normal, persist or eventually progress to invasive cervical cancer. The study of biomarkers that can distinguish CIN cases that will progress or regress has significant clinical value.
The conditions required for invasion of the cancer into the stromal tissue are, first, the ability of the cells to penetrate the underlying basement membranes and second, migration that involves adhesion to extracellular matrix constituents, such as laminins, collagens, and fibronectins. Laminins regulate cell adhesion, migration and differentiation, and is a family of glycoproteins of the extracellular matrix that constitute a major component of basement membranes. Laminin-5 is one of the isoforms of the laminin family and is composed of α3, β3 and γ2 chains, of which the γ2 is specific to laminin-5 Citation[11]. Decreased expression of the laminin-5γ2 chain has been found in different human cancer tissues such colon adenocarcinoma, ductal mammary carcinoma, squamous cell carcinoma of the skin, vulva, lung carcinoma, pancreatic adenocarcinoma, breast carcinoma, oral squamous cell carcinoma and tongue squamous cell carcinoma Citation[12], Citation[13]. Hao J et al. reported the decreases of expression of laminin-5γ2 in prostate cancer Citation[14]. Data from different groups suggested that laminin-5γ2 chain expression could be as a marker of invasive cancer in colon adenocarcinomas and in various types of squamous cell carcinomas Citation[12], Citation[15]. A correlation of laminin-5γ2 chain expression in the cytoplasm and/or the base membrane of cervical epithelium and with the grade of cervical dysplastic lesion has been reported earlier Citation[16]. It was also reported that laminin-5γ2 chain could be regarded as a marker for invasiveness of precancerous lesions in the lower anogenital tract Citation[17]. Skyldberg et al. suggested that immunohistochemically determined expression of laminin-5γ2 chain could be used as a sensitive marker to discriminate between non-invasive and invasive lesions Citation[18]. Hellman et al. showed that in patients with vaginal cancer, a high expression of the γ2 chain was significantly correlated with a short-term survival rate Citation[19].
p16INK4a, a tumor suppressor gene, is an element of the p16INK4a /cyclin D-cdk4/6/Rb pathway. It can induce G1 cell cycle arrest by inhibiting the phosphorylation of pRb by cdk4 and cdk6. Recent studies have shown that immunohistochemical staining with p16INK4a is a promising marker for dysplastic and malignant cervical epithelia Citation[20], Citation[21]. Klaes et al. found that overexpression of p16INK4a assists in the identification of high-risk HPV-related cervical squamous lesions Citation[20], and Sano et al. found that overexpression of p16INK4a was associated with HPV infection Citation[21], Citation[22]. In these studies it was suggested that p16INK4a appears to be useful for distinguishing immature squamous metaplasia form high-grade squamous intraepithelial lesion, where the former could pose as a morphologic differential diagnosis.
Proliferating cell nuclear antigens (PCNA) relates to DNA replication specifically detecting cells with active DNA replication, and it is universally used for evaluation of cell proliferation by immunohistochemistry. It appears PCNA is a good marker for proliferating cells regardless of the oncogenic capacity of the cells, as it does not distinguish dysplastic proliferation cells from normal proliferating cells. The aims of this study were 1) to investigate the expression of laminin-5γ2 chain, p16INK4a and PCNA in serial biopsies with histological diagnosis of different cervical lesions, 2) to evaluate the clinical significance of these proteins during the progression of cervical neoplasm.
Materials and methods
Cases selection
The material was obtained from women diagnosed on histology with CIN3 or invasive cervical cancer who received treatment in Umeå University Hospital. These patients were identified through the pathological registry between the years 1982 to 2000. The inclusion criterion was they were diagnosed with CIN3 or invasive cervical cancer and they have had previous biopsies taken representing different histological diagnosis of CIN. Thus, each case constitutes a series of 2 to 3 consecutive biopsy samples that represent normal squamous epithelium, CIN or invasive squamous cell carcinoma. In a histological section, tissue representing normal epithelium, different grades of CIN or invasive cancer can be present close to or adjacent to each other. In total in the entire material, there were 37 normal squamous cervical epithelium, 35 CIN lesions of different grades and 11 SCC of the cervix. These 15 cases with consecutive biopsies were used to study the importance of biomarkers in a human model for cervical cancer progression. The mean age of the 15 cases with CIN 3 and cancer diagnosis was 42.4 years (range: 24–58). The diagnosis of all cases had been reviewed and reconfirmed by two pathologists. Ethical approval to use the material was obtained for the study.
Immunohistochemistry
All specimens were formalin-fixed and paraffin-embedded. Serial sections (4 m thick) were cut and the end section was stained with H & E (haematoxylin and eosin) to ensure that the lesion was still present in the serial sections. The sections were processed for immunohistochemical staining. Sections were immersed in xylene to remove the paraffin followed by rehydration through graded alcohol. Epitope retrieval was performed by heating the sections for 10 minutes in 10 mM citrate buffer (pH 6.0) in a microwave oven. The sections were incubated in 0.75% hydrogen peroxide (H2O2) in methanol for a further 10 minutes to block all endogenous activity. This was followed by blocking of nonspecific binding of primary antibodies to epitopes by a preincubation step with 5% normal goat serum for 30 minutes at room temperature. The primary antibodies were then added and incubated at 4oC overnight. After washing thoroughly with PBS, the sections were incubated with secondary antibody conjugated to horseradish peroxidase (HRP) for 30 minutes at room temperature and developed with AEC + substrate-chromogen (DAKO Cytomation, Glostrup, Denmark). The sections were counterstained lightly with haematoxylin. Tissue sections containing colon cancer were used as positive controls for p16 INK4a staining and tissue sections of squamous cell cancer of the lung were used as positive controls for PCNA. Negative controls consist of PBS pH 7.4 in place of primary antibodies.
Preparation and characterization of polyclonal antibodies raised in rabbit against a fusion protein containing the C terminus of the laminin-5γ2 chain (containing amino acid residues # 1017–1178) and GST were performed according to methods described earlier Citation[23], Citation[18]. Mouse monoclonal F-12 anti-p16INK4a (Santa Cruz Biotechnology, CA, USA) and mouse monoclonal PC-10 anti-PCNA (Santa Cruz Biotechnology, CA, USA) were diluted 1:100. The secondary antibody was a goat anti-mouse diluted to 1:100 (Amersham Biosciences AB, Uppsala, Sweden). Nuclear staining was considered positive for p16INK4a and PCNA, while cells with distinct cytoplasmic immunoreaction were scored as laminin-5γ2 chain positive. Image analysis was done as described previously Citation[24]. Scoring of immunohistochemistry results were performed on the basis of both of the staining intensity and percentage of immunoreactive epithelial cells in the total of normal or neoplastic cells, as previously described Citation[25], Citation[26]. Staining score criteria for p16INK4a and PCNA was as following showed in percent (%):-(negative): (no expression); <20: weak staining ±; 20–30, weak or moderate staining +; 31–50 moderate or strong staining + + ; >50 strong staining + + +. The scores +, ++ and + + + were considered positive sample for p16INK4a and PCNA Citation[25–28]. The percentage of Laminin-5γ2 positive cells was estimated and categorized as follows and showed in percent:-(negative): no expression or <1%, +1–5%, ++ 6–30% and + + + >30%.
Statistical analysis
The data was analyzed with the SPSS program. Statistical analysis was based on ?2 test with Yates’ correction. An additional two-tailed Fisher's exact test was used only when the number of samples was less than ≤5. The t-test was used to analyze quantitative data. It was considered significant when p < 0.05.
Results
Positive expression rates of p16INK4a, laminin-5γ2 and PCNA among different cervical lesions
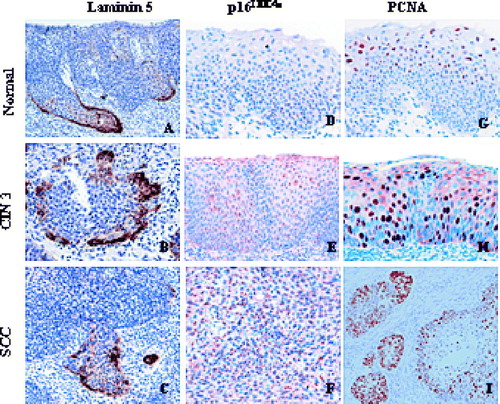
The expression of p16INK4a, laminin-5γ2 and PCNA were analyzed in 37 normal epithelium of the cervix, 17 CIN1/CIN2, 18 CIN3 and 11 invasive SCC in the biopsies of 15 women. The results of the immunohistochemical staining of p16INK4a, laminin-5γ2 and PCNA of these cases were as summarized in . The frequency of laminin-5γ2 chain staining seen at the basement membrane (BM + ) was found in 78.4% (29/37) of normal epithelium, in 41.2% (7/17) of CIN1/CIN2 and in 27.8% (5/18) of CIN3 (p < 0.001) (A). p16INK4a, the positive rate was significantly higher and it also increases with severity of lesion; CIN1/CIN2 has 64.7% (11/17) of positively stained cells, CIN3 77.8% (14/18) and SCC 86.2% (25/29) as compared to 29.7% (11/37) in normal squamous epithelium (p < 0.001). However, the difference between the various histological diagnoses, CIN1/CIN2, CIN3 and invasive cancer was not significant for p16INK4a expression (p > 0.05). As for laminin-5γ2 chain expression at the basement membrane of cancerous lesions was negative. No expression was evident in the stromal cells. In comparison, the cytoplasmic staining of laminin-5γ2 was detected in 2.7% (1/37) of normal epithelium, 17.7% (3/17) of CIN1/CIN2 (p < 0.05), 31.6% (6/18) of CIN3 (p < 0.01), and 89.7% (26/29) in invasive cancers (p < 0.001) (A). PCNA expression was found in 10.8% (4/37) of normal epithelium, in 35% (6/17) of CIN1/CIN2, 72.2% (13/18) of CIN3 and 93.1% (27/29) of SCC. The rate of PCNA expression between the various histological diagnoses was significantly increased and it increase with the severity of the disease (p < 0.001) (B).
Figure 1. A. The frequency of laminin-5γ2 chain staining seen at basement membrane and cytoplasm of normal epithelium, different stages of CIN and invasive cervical cancer. B. The frequency of p16INK4a, PCNA expression in samples from normal epithelium, different CIN stages, and invasive cervical cancer.
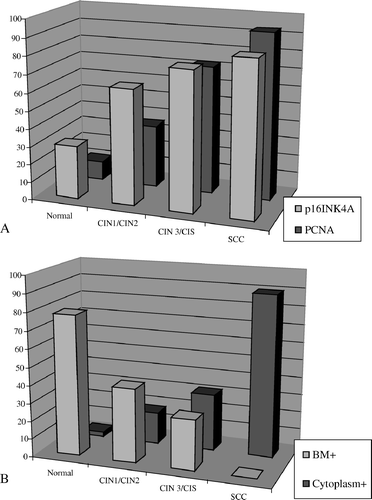
Table I. Expression of Laminin-5γ2, p16INK4a and PCNA in the progression of cervical neoplasia
Expression and co-expression pattern of p16INK4a, laminin-5γ2 and PCNA in the cells of different cervical lesions
Laminin-5γ2 chain expression can be either intra- or extracellular. None of the cells in the normal epithelium expressed any intracytoplasmic laminin-5γ2 chain. Instead, laminin-5γ2 chain was exclusively extracellular in the basement membrane of normal epithelium. With increasing severity of dysplasia, laminin-5γ2 chain expression tends to be more intracellular in the cytoplasm of abnormal cells while its expression at the basement membrane gradually disappears. In tumor cells, laminin-5γ2 chain immunoreactivity was exclusively intracytoplasmic and most of these cells stain moderate to strong. However, the staining intensity appears to decrease to low or to moderate in some of the high-grade CIN lesions. The positively stained cells of high grade CIN were restricted to those projecting into the stroma, and in SCC, the positive cells line the invasion front of the lesion. (A, B, C). Unlike laminin-5γ2 chain expression, the expression of p16INK4a was predominately nuclear and it stained cells located in the basal layer of normal epithelium, or in the lower 1/3 layer of CIN1/CIN2, or the entire epithelial layer of CIN3. In SCC, p16INK4a expression was evident in cells occupying the entire lesion invading into the stroma (D, E, F). In normal epithelium, few cells stained positive for PCNA and they were located primarily in the basal layer. In CIN1/CIN2, the expression of PCNA was restricted in the lower 1/3 layer to the upper 1/3 layer while in high grade CIN diagnosis PCNA was present in the entire thickness of the epithelium. Similarly for SCC, the positive cells were seen either at the outer edge of the lesion or they occupy the entire area of the invading front of the tumor (G, H, I).
Figure 2. Expression of laminin-5γ2 chain, p16INK4a and PCNA in samples from normal epithelium, different CIN stages, and invasive cervical cancer.
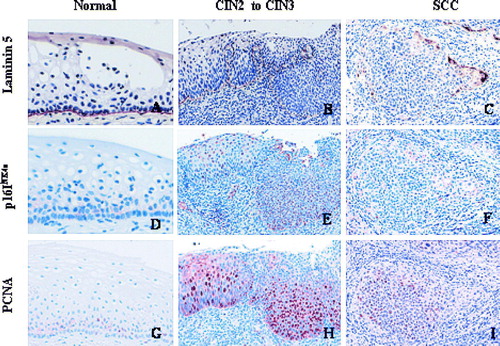
We also looked at the co-expression of p16INK4a, laminin-5γ2 chain and PCNA in the epithelia of these biopsies. Laminin-5γ2 chain staining was primarily positive extracellularly in the basement membrane, and its staining intensity decreases from strong to weak, or disappear with increasing disease severity. p16INK4a and PCNA expression were both nuclear and they were either negative or sporadically positive in the basal layer of normal epithelium in different biopsies (A, B, C). In progressing lesions, the staining intensity and the number of p16INK4a and PCNA positive cells were also increased except in some cancer cases where the staining pattern were variable within the lesion (D, E, F). Cytoplasmic laminin-5γ2 chain positive cells in SCC were located more at the front or tip of the cancer lesion where as p16INK4a expression was weakly positive and PCNA was negative in the same area (G, H, I).
p16INK4a, laminin-5γ2 chain and PCNA expression during progression of cervical neoplasia
The significance the time interval taken for progression from normal or low grade CIN to high grade CIN or SCC in relation to the expression of laminin-5γ2 chain, p16INK4a and PCNA was evaluated. Eight of these cases progressed from normal to CIN or SCC, one case progressed from CIN1/CIN2 to CIN3 and six cases progressed from CIN to SCC. The expression of these proteins and patient's age, diagnosis and the time the biopsy was taken were shown in . Basement membrane (BM + ) staining for laminin-5γ2 chain was 69.6% (7/8) for normal epithelium and 37.5% (6/16) in CIN while the intracytoplasmic staining for laminin-5γ2 chain was absent in (0/8), 12.5% (2/16) and 90.9% (10/11) of normal epithelium, CIN and SCC, respectively. Co-expression of p16 INK4a and PCNA was seen in 85.7% (30/35) of samples. The combination of the three markers; laminin-5γ2 chain BM + /p16INK4a−/PCNA− was found in 5 of 8 (62.5%) samples with normal epithelium, laminin-5γ2 chain BM − /p16INK4a+/PCNA+ was found in 10 of 16 in CIN (62.5%). Only two samples with CIN had intracytoplamic staining for laminin-5γ2 chain where p16INK4a and PCNA expression were also positive simultaneously. Laminin-5γ2 chain Cyto + /p16INK4a +/PCNA+ expression was seen in 63.6% (7/11) with SCC (results not shown). The co-expression of laminin-5γ2 chain, p16INK4a, and PCNA during development of cervical neoplasia was showed in . The cases that were laminin-5γ2 chain BM − /p16INK4a+/PCNA+ seemed to have the shortest interval time (average time 46.8±36.3 months) for progression. However, the cases with combination of laminin-5γ2 chain BM + /p16INK4a−/PCNA− have the longest time interval (average time 110.2±52.7 months). A significant difference was seen between the two combinations (p < 0.05).
Table II. Co-rexpression of Laminin 5, p16INK4a, PCNA and the interval time for progression of cervical neoplasia
Discussion
The natural history of cervical cancer involves a number of events from the time of early detection of neoplasia to invasive cancer. The entire transformation process could take 13 to 20 years, affecting women in their mid forties or early fifties. HPV persistence has been established as a necessary cause for cervical cancer development Citation[29] but HPV test alone has a low positive predictive value Citation[30]. Several studies had shown that as the disease progressed from low grade CIN to high grade CIN or to invasive cancer, there was evidence of telomere attrition, an accumulation of genetic aberrations ultimately leading to aneuploidy, genomic amplifications, over expression of oncogenes, disruption of the cell cycle mechanism and imbalance of cell division Citation[31–34].
The markers used in this study, laminin-5γ2 chain, p16INK4a and PCNA were targeted to properties for tumor invasion, cell cycle disruption as a consequent of loss of p53 and/or pRB control, and as a result an increase in cellular proliferation. Kohlberger et al. reported basement membrane staining for laminin-5γ2 chain to be 69.4% to 77.8% in CIN1 to CIN3, and negative in normal epithelium and invasive cancer Citation[16]. However, in our study, we found a reduction of basement membrane staining for laminin-5γ2 chain from 78.4% in normal epithelium to 41.2% in CIN1/CIN2, 27.8% in CIN3 and negative in SCC. Kohlberger et al. found no intracytoplasmic staining for laminin-5γ2 chain in normal epithelium of the cervix, while 2.8%, 20% and 30.9% of CIN1, CIN2 and CIN3 were laminin-5γ2 chain expression positive, respectively. We also found intracytoplasmic staining for laminin-5γ2 chain increased with cervical lesion progression Citation[16]. This suggests that the loss of laminin-5γ2 chain in the basement membrane and the overexpression of laminin-5γ2 chain in the cytoplasm may be important steps in tumor cell invasion of cancer. Such changes in the expression pattern from being extracellular to intracellular of laminin-5γ2 chain and being more evident in cells located at the invasion front of high grade CIN and invasive cancers further indicate that this marker is an excellent marker for progression to invasion. Alteration in laminin-5γ2 chain expression and its tissue distribution are able to reflect changes in epithelial-mesenchymal adhesion and integrity. Such alterations have been reported in oral squamous carcinoma, breast cancer, bladder carcinoma and invasive cervical cancer Citation[18], Citation[35–37] which we also see in women with serial biopsies of lesions progressing from normal/low grade CIN to CIN3 and invasive cervical cancer. Furthermore, an overexpression of the laminin-5γ2 chain was associated with poor patient prognosis Citation[38] and results from our previous study indicated that the γ2 chain of the laminin-5 marker is more commonly expressed in squamous cell carcinoma of the cervix than in adenocarcinoma Citation[39].
Previous studies have shown that p16INK4a is strongly expressed in almost all cervical cancers. Nielsen et al. demonstrated p16 INK4a staining in some areas of squamous metaplasia with only relatively few Ki-67 positive cells in the same area Citation[40]. Interestingly, we found co-expression of p16 INK4a and PCNA in the premalignant lesions and invasive cancer in our study. Moreover, the areas positive for p16 INK4a and PCNA overexpression were negative or only sporadically positive for laminin-5γ2 chain in the cervical cancer lesions of our material. Laminin-5γ2 chain positive cells were often located in the front of invasive cancer lesions, where p16 INK4a showed weak expression or was negative while PCNA had no expression at all within the same area. However Natarajan et al. reported 15 of 17 areas of dysplasia, microinvasion, and superficial margins of advanced oral squamous cell cancers had both p16 INK4a and laminin-5γ2 chain expressed together, and in 13 of these 15 lesions co-localization of laminin-5γ2 chain and p16INK4a was obvious Citation[41]. Cells at the epithelial-stromal interfaces of invasive SCCs lying deep in the dermal tissue typically expressed neither protein. The authors considered that p16INK4a and laminin-5γ2 chain typically becomes expressed coordinately in cells at locations just proceeding and at the time of initial invasion into connective tissue. The difference between our data could be related to the different tissue type and the presence of HPV infection in our material.
Preinvasive lesions of the cervix are frequent, especially in young women, with a peak in incidence between the age of 25 and 40 years Citation[42]. It was suggested that approximately 12.2% of all CIN3, when left untreated, would develop into invasive cancer with a mean duration of 13.3 years disease for the in-situ stage Citation[43]. There is yet no reliable way to predict if a CIN lesion will progress to invasive cancer or if it will remain stable or regress to normal. Nordemar et al. noted that all six carcinoma-in-situ (CIS) lesions that progressed to invasive cancer within a follow-up time of five years, were laminin-5γ2 chain positive (100%), whereas only 10 of 27 lesions that did not progress, were positive (37%) (p < 0.01) in the larynx Citation[44]. The authors suggest that a positive laminin-5γ2 chain laryngeal CIS lesion indicates a high risk for progression to invasive cancer. Skyldberg et al. also demonstrated that all microinvasive cancers and frankly invasive cancers had positive cytoplasmic staining for laminin-5γ2 chain, whereas only 11 of 32 lesions with CIN3 showed positive staining for laminin-5γ2 chain in the cells close to the basement membrane Citation[18]. Most CIN1 and CIN2 lesions were negative for laninin-5γ2 chain at the basement membrane with exception to cases that progressed from high grade CIN to invasive cancer. In the present study, biopsies that were basement membrane negative for laminin-5γ2 chain but with overexpression of p16INK4a and PCNA progressed to CIN or SCC require a shorter time for progression than those with positive basement membrane for laminin-5γ2 chain while the epithelium was negative for p16 INK4a and PCNA expression. In our previous study we reported the mean time taken for progression if only p16 INK4a was present, namely 64.2 months Citation[24] while a combination of expression of these three markers used in this study had a time interval of gave a shorter mean time interval of 46.8 months.
In conclusion, in this study we could see that the loss of basement membrane and increased cytoplasmic staining for laminin-5γ2 chain is related to the severity of cervical neoplasia. Laminin-5γ2 chain strongly stained cells that might be involved with invasion of cancer cells. Cases with the combination of laminin-5γ2 chain BM − /p16 INK4a/PCNA+ have a shorter interval time for the progression of cervical neoplasia. Hence, the co-expression of laminin-5γ2 chain and p16 INK4a and PCNA simultaneously may be valuable for the prediction of cases that will progression.
We would like to thank Prof. Anders Hjerpe for reviewing and reconfirming of the histopathological diagnosis of the material. This study was supported by grants from the Swedish Cancer Foundation, the Swedish Medical Research Council, the Swedish Cancer Society and county council of Stockholm.
References
- zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians 1999; 111: 581–7
- Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst 1995; 87: 796–802
- Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348: 518–27
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189: 12–9
- Andersson S, Rylander E, Larsson B, Strand A, Silfversvard C, Wilander E. The role of human papillomavirus in cervical adenocarcinoma carcinogenesis. Eur J Cancer 2001; 37: 246–50
- Andersson S, Rylander E, Larson B, Sigurdardottir S, Backlund I, Sallstrom J, et al. Types of human papillomavirus revealed in cervical adenocarcinomas after DNA sequencing. Oncol Rep 2003; 10: 175–9
- Sasieni P, Adams J. Changing rates of adenocarcinoma and adenosquamous carcinoma of the cervix in England. Lancet 2001; 357: 1490–3
- Herbert A, Singh N, Smith JA. Adenocarcinoma of the uterine cervix compared with squamous cell carcinoma: A 12-year study in Southampton and South-west Hampshire. Cytopathology 2001; 12: 26–36
- Tenti P, Romagnoli S, Silini E, Zappatore R, Spinillo A, Giunta P, et al. Human papillomavirus types 16 and 18 infection in infiltrating adenocarcinoma of the cervix: PCR analysis of 138 cases and correlation with histologic type and grade. Am J Clin Pathol 1996; 106: 52–6
- Vizcaino AP, Moreno V, Bosch FX, Munoz N, Barros-Dios XM, Parkin DM. International trends in the incidence of cervical cancer: I. Adenocarcinoma and adenosquamous cell carcinomas. Int J Cancer 1998; 75: 536–45
- Ryan MC, Tizard R, VanDevanter DR, Carter WG. Cloning of the LamA3 gene encoding the alpha 3 chain of the adhesive ligand epiligrin. Expression in wound repair. J Biol Chem 1994; 269: 22779–87
- Sordat I, Bosman FT, Dorta G, Rousselle P, Aberdam D, Blum AL, et al. Differential expression of laminin-5 subunits and integrin receptors in human colorectal neoplasia. J Pathol 1998; 185: 44–52
- Soini Y, Maatta M, Salo S, Tryggvason K, Autio-Harmainen H. Expression of the laminin gamma 2 chain in pancreatic adenocarcinoma. J Pathol 1996; 180: 290–4
- Hao J, Yang Y, McDaniel KM, Dalkin BL, Cress AE, Nagle RB. Differential expression of laminin 5 (alpha 3 beta 3 gamma 2) by human malignant and normal prostate. Am J Pathol 1996; 149: 1341–9
- Pyke C, Salo S, Ralfkiaer E, Romer J, Dano K, Tryggvason K. Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res 1995; 55: 4132–9
- Kohlberger P, Beneder C, Horvat R, Leodolter S, Breitenecker G. Immunohistochemical expression of laminin-5 in cervical intraepithelial neoplasia. Gynecol Oncol 2003; 89: 391–4
- Nordstrom B, Einhorn N, Silfversward C, Sjovall K, Tryggvason K, Auer G. Laminin-5 gamma 2 chain as an invasivity marker for uni- and multifocal lesions in the lower anogenital tract. Int J Gynecol Cancer 2002; 12: 105–9
- Skyldberg B, Salo S, Eriksson E, Aspenblad U, Moberger B, Tryggvason K, et al. Laminin-5 as a marker of invasiveness in cervical lesions. J Natl Cancer Inst 1999; 91: 1882–7
- Hellman K, Hellstrom AC, Silfversward C, Salo S, Aspenblad U, Nilsson B, et al. Cancer of the vagina: Laminin-5gamma2 chain expression and prognosis. Int J Gynecol Cancer 2000; 10: 391–6
- Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer 2001; 92: 276–84
- Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Immunohistochemical overexpression of p16 protein associated with intact retinoblastoma protein expression in cervical cancer and cervical intraepithelial neoplasia. Pathol Int 1998; 48: 580–5
- Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol 1998; 153: 1741–8
- Salo S, Haakana H, Kontusaari S, Hujanen E, Kallunki T, Tryggvason K. Laminin-5 promotes adhesion and migration of epithelial cells: Identification of a migration-related element in the gamma2 chain gene (LAMC2) with activity in transgenic mice. Matrix Biol 1999; 18: 197–210
- Wang JL, Zheng BY, Li XD, Angstrom T, Lindstrom MS, Wallin KL. Predictive significance of the alterations of p16INK4A, p14ARF, p53, and proliferating cell nuclear antigen expression in the progression of cervical cancer. Clin Cancer Res 2004; 10: 2407–14
- Sano T, Masuda N, Oyama T, Nakajima T. Overexpression of p16 and p14ARF is associated with human papillomavirus infection in cervical squamous cell carcinoma and dysplasia. Pathol Int 2002; 52: 375–83
- Klussmann JP, Gultekin E, Weissenborn SJ, Wieland U, Dries V, Dienes HP, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol 2003; 162: 747–53
- Lindstrom MS, Klangby U, Inoue R, Pisa P, Wiman KG, Asker CE. Immunolocalization of human p14(ARF) to the granular component of the interphase nucleolus. Exp Cell Res 2000; 256: 400–10
- Herbsleb M, Knudsen UB, Orntoft TF, Bichel P, Norrild B, Knudsen A, et al. Telomerase activity, MIB-1, PCNA, HPV 16 and p53 as diagnostic markers for cervical intraepithelial neoplasia. Apmis 2001; 109: 607–17
- Wallin KL, Wiklund F, Angstrom T, Bergman F, Stendahl U, Wadell G, et al. Type-specific persistence of human papillomavirus DNA before the development of invasive cervical cancer. N Engl J Med 1999; 341: 1633–8
- von Knebel Doeberitz M. New molecular tools for efficient screening of cervical cancer. Dis Markers 2001; 17: 123–8
- Zhang A, Wang J, Zheng B, Fang X, Angstrom T, Liu C, et al. Telomere attrition predominantly occurs in precursor lesions during in vivo carcinogenic process of the uterine cervix. Oncogene 2004; 23: 7441–7
- Keating JT, Cviko A, Riethdorf S, Riethdorf L, Quade BJ, Sun D, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus–related cervical neoplasia. Am J Surg Pathol 2001; 25: 884–91
- Zhang A, Maner S, Betz R, Angstrom T, Stendahl U, Bergman F, et al. Genetic alterations in cervical carcinomas: Frequent low-level amplifications of oncogenes are associated with human papillomavirus infection. Int J Cancer 2002; 101: 427–33
- Skyldberg B, Fujioka K, Hellstrom AC, Sylven L, Moberger B, Auer G. Human papillomavirus infection, centrosome aberration, and genetic stability in cervical lesions. Mod Pathol 2001; 14: 279–84
- Kosmehl H, Berndt A, Strassburger S, Borsi L, Rousselle P, Mandel U, et al. Distribution of laminin and fibronectin isoforms in oral mucosa and oral squamous cell carcinoma. Br J Cancer 1999; 81: 1071–9
- Henning K, Berndt A, Katenkamp D, Kosmehl H. Loss of laminin-5 in the epithelium-stroma interface: An immunohistochemical marker of malignancy in epithelial lesions of the breast. Histopathology 1999; 34: 305–9
- Hindermann W, Berndt A, Haas KM, Wunderlich H, Katenkamp D, Kosmehl H. Immunohistochemical demonstration of the gamma2 chain of laminin-5 in urinary bladder urothelial carcinoma. Impact for diagnosis and prognosis. Cancer Detect Prev 2003; 27: 109–15
- Moriya Y, Niki T, Yamada T, Matsuno Y, Kondo H, Hirohashi S. Increased expression of laminin-5 and its prognostic significance in lung adenocarcinomas of small size. An immunohistochemical analysis of 102 cases. Cancer 2001; 91: 1129–41
- Andersson S, Hellström A-C, Ångström T, Stendahl U, Auer G, Wallin K-L. The Clinicopathological significance of laminin-5 gamma 2 chain expression in cervical squamous- and adenocarcinoma. 2005 (in press).
- Nielsen GP, Stemmer-Rachamimov AO, Shaw J, Roy JE, Koh J, Louis DN. Immunohistochemical survey of p16INK4A expression in normal human adult and infant tissues. Lab Invest 1999; 79: 1137–43
- Natarajan E, Saeb M, Crum CP, Woo SB, McKee PH, Rheinwald JG. Co-expression of p16(INK4A) and laminin 5 gamma2 by microinvasive and superficial squamous cell carcinomas in vivo and by migrating wound and senescent keratinocytes in culture. Am J Pathol 2003; 163: 477–91
- Wright TC, Sun XW, Koulos J. Comparison of management algorithms for the evaluation of women with low-grade cytologic abnormalities. Obstet Gynecol 1995; 85: 202–10
- Reid REA. Preinvasive disease. Practical gynecologic oncology2nd ed, J Berek, NF Hacker. Williams & Wilkins, Baltimore 1994
- Nordemar S, Kronenwett U, Auer G, Hogmo A, Lindholm J, Edstrom S, et al. Laminin-5 as a predictor of invasiveness in cancer in situ lesions of the larynx. Anticancer Res 2001; 21: 509–12