Abstract
The aim of this study was to develop and evaluate 111In- and 99mTc-labeled derivatives of albumin nanocolloid (NC) for dual-label lymphoscintigraphy to allow simultaneous comparison of lymphatic flow from different tissue planes draining a tumour bed for accurate identification of sentinel lymph nodes (SLN). Using the chelator, p-isothiocyanatobenzyl-1,4,7,10-tetraazacyclododecane-1,4,7,10- tetraacetic acid (DOTA), 111In-DOTA-NC and 99mTc-DOTA-NC were compared in vitro with respect to stability of labeling, colloidal status and particle size, then in vivo by measuring their clearance rates from a subcutaneous injection depot. 111In-DOTA-NC and 99mTc-DOTA-NC were indistinguishable on the basis of in vitro criteria. Their in vivo clearance rates, however, were disparate (0.0015 to 0.075 min−1 for 111In and 0.0072 to 0.067 min−1 for 99mTc), 111In being faster in three studies and markedly slower in three. This demonstrates that even when dual-labeled radiotracers behave identically in vitro, they will not necessarily do so in vivo. Further work is needed to develop dual-labeled NC.
Sentinel lymph node (SLN) identification is becoming established as a central technique in the management of early breast carcinoma Citation[1]. Its role is also being explored for other superficial cancers that spread preferentially by lymphatics such as melanoma Citation[2], squamous cell carcinoma of the head and neck Citation[3] and vulva Citation[4]. The technique is also applicable to the staging of intracavitatory neoplasms such as lung Citation[5] and colon Citation[6] and in the correct clinical setting may be more accurate than any radiological staging technique including FDG-PET Citation[7].
The success of the technique depends on appropriate placement of the SLN tracer in relation to the tumour and its draining lymphatics. In melanoma, intradermal placement of tracer accurately identifies the SLN from the dermal primary tumour Citation[8], Citation[9]. Deeper placed primaries, however, may spread through lymphatic drainage pathways in multiple tissue planes, making decisions regarding the ideal placement of tracer injections more complex. This is illustrated with respect to breast carcinoma by the extensive debate within the literature concerning alternative injection sites Citation[10–12], an issue that is now being addressed by several very large studies Citation[13].
In order to resolve this issue, it would be valuable to have a dual isotope technique that would allow simultaneous interrogation of alternative planes taken by tracers that differ only in their radioisotope label. The tissue plane preferred by cancer cells metastasising via lymphatics could then be established by comparing the distributions of radiolabel and metastatic cancer cells within the dissected lymph nodes. This in turn would allow the ideal site of injection to be established in a relatively small group of patients for each tumour group and enable application of the SLN technique to other regions of surgical oncology.
Such a dual-isotope technique would require demonstration that the lymphoscintigraphic tracers behave identically in vivo so that any difference in their nodal distribution could be correctly assigned to differences in the lymphatic drainage pathways rather than any inherent difference in the tracer particles themselves. Prior to such in vivo testing, the radiotracers would need to fulfil the following in vitro criteria: stable binding of both radiometals, minimal ‘cross-over’ of label during incubation, maintenance of the colloidal status of the product after conjugation, purification and labeling, and comparability of molecular or particulate size.
99mTc-labeled albumin nanocolloid (NC) is the most widely used tracer for SLN localisation in Europe and would therefore be the preferred agent for a dual isotope technique if labeling with a second radionuclide could be successfully developed for it. NC consists of microaggregated human serum albumin (HSA) with 95% of the colloidal particles ≤80 nm in diameter. A previous attempt to label native HSA with 111In and 99mTc using DTPA chelation was unsuccessful as the 111In label was unstable Citation[14]. The purpose of the current study, therefore, was to develop a dual-isotope method using an alternative chelating agent, p-isothiocyanatobenzyl-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (p-SCN-Bz-DOTA), as chelator and evaluate it in vitro then in vivo by measuring differential depot clearance rates following interstitial injection in human volunteers. p-SCN-Bz-DOTA is a highly effective bifunctional chelating agent. The isothiocyanate portion of the p-SCN-Bz-DOTA conjugates to the ε-amino group of lysine residues and the DOTA group provides a closed ring or ‘cage’ in which the radiometal, either 111In or 99mTc, is contained, making the conjugated protein theoretically suitable for the intended purpose.
Methods
Preparation of radiopharmaceuticals
111In-DOTA-NC
The bifunctional chelating agent, p-SCN-Bz-DOTA, was conjugated to human albumin nanocolloid as follows. A batch of four vials of commercial NC (Nanocoll, Amersham Health), each containing 0.5 mg microaggregated HSA, was reconstituted with 1.0 ml normal saline. The pooled protein (2 mg) was transferred to a plastic test tube after which 0.5 ml of 0.1 M sodium bicarbonate (pH 7.8) buffer was added and the mixture allowed to incubate for one hour at room temperature. The protein was derivatised at p-SCN-Bz-DOTA to protein molar ratio of 4:1 by the addition of 16 µl of p-SCN-Bz-DOTA (Macrocyclics) from a stock solution of 5 mg/ml in DMSO to a stirred suspension of NC. The preparation was left to incubate overnight at room temperature. To purify the material, 0.5-ml aliquots of conjugated protein were transferred to Centricon filters (Amicon YM30, 30,000-Da molecular weight cut-off) and centrifuged at 1500 g for 150 min. After two volume washes with saline, the protein concentrated by the filters was collected by reverse centrifugation and the volume made up to 1 ml with saline. The Centricon-filtered protein was further purified on a Sephadex G-25 PD10 column (Amersham Pharmacia Biotech) to remove any unbound p-SCN-Bz-DOTA. Briefly, 1 ml of the conjugate was applied to the top of the column and eluted with 10×1-ml fractions of saline. The UV absorbance at 298 nm of each fraction was measured using a spectrophotometer. Aliquots (0.1 mg/0.1 ml) of the two fractions with the highest protein concentration were separately labeled with 111In-acetate as follows. Five µl (up to 3 MBq) of 111In-chloride (Tyco Healthcare) were mixed thoroughly with 20 µl of 1 M sodium acetate (pH 5.0) in a microcentrifuge tube. After 2 min, 100 µl of the derivatised NC were added to the tube and the mixture was allowed to incubate at room temperature for 15–30 min. The radiochemical purity (RCP) of the complex was determined using instant thin-layer chromatography (ITLC-SG, Pall Gelman Sciences) as stationary phase and acid citrate dextrose (ACD) as mobile phase. 111In-labeled NC remained at the origin (Rf = 0.0) and free 111In migrated with the solvent front (Rf = 1.0). Column recovery was approximately 50% and the concentration of conjugated NC, measured using UV spectrophotometer, was 1 mg/ml. The two fractions containing derivatised material were combined, passed through a sterile 0.22-µm membrane filter into a sterile vial, and stored at 2–8°C. An identical procedure was followed for a second batch of four vials of NC. For in vitro and in vivo experiments, 111In-DOTA-NC was prepared and analysed as described above. Retrospective sterility testing was negative.
99mTc-DOTA-NC
Aliquots of 0.1 mg (100 µl) of derivatised DOTA-NC (above) were labeled with ∼100 MBq 99mTc-pertechnetate obtained from a generator with less than 24 hours ingrowth (Amertec II, Amersham Health) using 8 µg stannous chloride as reducing agent. RCP was measured on ITLC-SG strips developed with saline. 99mTc-DOTA-NC remained at the origin (Rf = 0.0) and free 99mTc migrated with the solvent front (Rf = 1.0). Retrospective sterility testing was negative.
Stability of labelling
The stability of 111In-DOTA-NC was evaluated by incubation in saline and DTPA challenge (200-fold molar excess) performed at 37°C. RCP measurements were taken every two hours up to eight hours. The stability of 99mTc-DOTA-NC was evaluated by incubation in serum and cysteine challenge (500-fold molar excess) performed at 37°C. RCP measurements were taken every two hours up to eight hours. Different challenge conditions were used for the two labels because of their differing chemistries.
Colloidal status
Colloidal status of the labeled 111In-DOTA-NC was evaluated by ITLC-SG (saturated with HSA) developed in ethanol:ammonium hydroxide:water (2:1:5). In this system, labeled albumin moves while colloids remain at the origin.
‘Cross-over’ of radiolabels
111In-DOTA-NC and 99mTc-DOTA-NC were incubated together in a mixture for four hours at room temperature. RCP of each compound in the mixture was measured immediately after preparation and after the period of four hours of incubation.
Particle sizing
The particle size of modified NC was compared to native NC by filtration through polycarbonate membranes. The labeled preparations were diluted with saline to a radioactivity concentration of ∼500 kBq/ml. Using a 1-ml syringe, 0.1 ml of the preparation was carefully injected into a 100-nm filter in a holder. A 2-ml volume of saline was passed through the filter followed by 2 ml of air. The activities in the filter and filtrate were then assayed in a gamma scintillation counter. The filtrate activity was corrected for any unbound activity found on ITLC, and finally particle sizes were expressed as % passing through a 100-nm filter and compared to a standard preparation of 99mTc-NC. The manufacturer states that >95% of the colloidal particles are ≤80 nm in diameter.
In vivo comparison of 111In-DOTA-NC and 99mTc-DOTA-NC kinetics
Four normal subjects were recruited after giving written fully informed consent. This study was approved by the Administration of Radioactive Substances Advisory Committee of the United Kingdom and the local Research Ethics Committee. Two volunteers took part on two occasions each, giving a total of six studies.
A 0.2-ml injection consisting of a cocktail of 0.5 MBq 111In-DOTA-NC and 1.0 MBq 99mTc-DOTA-NC was administered subcutaneously into the webspace between the second and third metacarpals within three hours of preparation. The clearance rates of the two tracers from the site of depot injection were measured with a collimated sodium iodide scintillation detector as previously described Citation[15–17]. The method was adapted to measure the two radionuclides simultaneously, with one window centred on the 140 keV peak for 99mTc, and a second window including the 173 and 247 keV peaks for 111In. The cross-talk correction factors were established from measurements on a phantom hand, using similar activities and scattering conditions to those in previous studies Citation[15–17]. Measurements were made at approximately 15 min intervals for three to five hours. Counts rates within the two windows were corrected for background, cross-talk and physical decay of the radionuclides. The clearance rates were quantified by least squares fitting of a monoexponential function to the individual data points, and expressed with units of min−1.
Results
In vitro studies
Both radiotracers were effectively stable in vitro, releasing less than 5% of the radiolabel in eight hours despite DTPA and cysteine challenges ().
Table I. Release of attached label (% of initial) from 111In- or 99mTc-DOTA-NC as a function of time of incubation at 37°C (mean of 2 batches).
There was very little variation in particle size range between 99mTc-DOTA-NC and 111In-DOTA-NC, and the particle sizes of both 99mTc-DOTA-NC and 111In-DOTA-NC were comparable to ‘native’ 99mTc-NC () and similar to the manufacturer's specifications for 99mTc-NC.
Table II. Particle sizing by membrane filtration (n = 1).
There was no detectable cross-over of radiolabel between the radiopharmaceuticals over a four hour incubation period (data not shown).
More than 95% of the activity in two determinations was confirmed as colloidal when evaluated by thin-layer chromatography with HSA-saturated ITLC-SG.
In vivo studies
The clearances of 111In-DOTA-NC and 99mTc-DOTA-NC over three to five hours following subcutaneous injection as a cocktail broadly conformed to a monoexponential function ( and ) with depot clearance rates ranging from 0.0015 to 0.075 min−1 and 0.0072 to 0.067 min−1, respectively. In general, however, there was poor agreement between the two radiotracers with respect to these clearance rates which could not be explained on the basis of variations in RCP (). Indeed, relative clearance rates in two subjects studied on the same day using the same preparations of tracers (studies 1 and 2) being widely disparate. Individual patient factors would also be unlikely to be responsible for this disparity as repeated studies on the same subjects (1 & 4 and 2 & 3) also showed wide variation in clearance rates of the two tracers.
Figure 1. Comparative depot clearance rates of 99mTc and 111In following co-injection of 99mTc-DOTA-NC and 111In-DOTA-NC into the second web space of the hand in six normal volunteers. The data were corrected for cross-talk and physical decay of the radionuclide. In three studies there was good agreement between the respective clearance rates but in three others there was clear divergence. In two volunteers studied twice there was poor reproducibility of clearance rates.
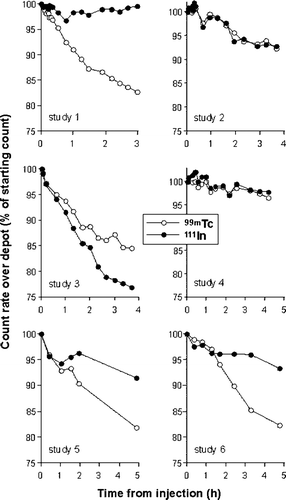
Table III. Clearance rate constants of 111In-DOTA-NC and 99mTc-DOTA-NC following subcutaneous injection into the 2nd webspaces of the hands of four normal volunteers, of whom two (studies 1&4 and 2&3) were each studied on two separate occasions. Radiochemical purity (RCP, %) of the injected material is also shown. Studies 1 and 2 were performed on the same day using identical material, as were studies 5 and 6.
Discussion
With any new radiopharmaceutical, satisfactory in vitro performance is essential but this does not necessarily predict success in vivo. On the basis of successful in vitro testing of 99mTc-DOTA-NC and 111In-DOTA-NC, we were justified in going ahead to assess the in vivo behaviour of these two radiotracers. Although the two radiopharmaceuticals behaved more or less identically in vitro, demonstrating good stability of labeling and uniform particle size, they gave significantly disparate kinetics in vivo, making them unsuitable for quantifiable dual isotope lymphoscintigraphy. Mitterhauser et al Citation[18] recently reported direct labeling of NC with 111In, which they compared with 99mTc-NC but only on the basis of in vitro criteria. The current study illustrates that before claims for such success can be accepted, the tracers must be shown to behave identically in vivo as well as in vitro.
Human immunoglobulin G (HIG) and HSA are alternatives to NC for lymphoscintigraphy Citation[19–21]. 99mTc-HIG, however, is inferior to 99mTc-NC in one important respect in relation to SLN identification and that is a lower lymph node extraction fraction which we have recently shown to be ∼0.45 for 99mTc-HIG compared to ∼0.7 for 99mTc-NC Citation[22]. Although it has not been measured, the extraction fraction of HSA is likely to be even lower because HSA lacks an Fc portion for potential binding to lymph node macrophage Fc receptors. In vitro criteria demonstrated similarity between 111In-HSA and 99mTc-HSA and between 111In-HIG and 99mTc-HIG Citation[14]. However, unlike both NC in the current study and previously with HSA Citation[14], 111In-HIG and 99mTc-HIG have been shown to give almost identical in vivo kinetics in addition to identical in vitro behaviour Citation[15–17], with very similar clearance rates from a subcutaneous web space injection depot (same protocol as used in the current study) and subsequent appearance rates in central blood, validating HIG for dual-isotope lymphoscintigraphy. Interestingly, a more sensitive in vivo benchmark of behaviour, namely recovery in venous blood ipsilateral to the side of injection, was still able to distinguish between these two HIG tracers Citation[17].
The disparity between the in vitro and in vivo results in the current study remains unexplained. In three of the six subject studies (2, 3 and 4) 111In-DOTA-NC cleared faster than 99mTc-DOTA-NC, whilst in the remaining three (1, 5 and 6) the converse was the case, and markedly so. This indicates that the relationship between the two clearance rates is not a simple one. It may be that under physiological conditions, the NC particles associate with each other and form larger aggregates that are slower to be taken up into the lymphatics, with the 111In-labeled agent in this respect behaving differently to the 99mTc agent. Variations in clearance as a result of the small variations in RCP can be discounted from the complete lack of correlation between the two. Indeed, the same preparations given to different subjects on the same day gave completely different clearance rates (). The variability in relative clearance rates may also have been related to subject factors; although the lack of any reproducibility between consecutive studies carried out on the same subjects at different times () would argue against this.
Conclusion
This study emphasises the importance of following up favourable in vitro data with in vivo confirmation before any new dual-labeled lymphoscintigraphic agent can be used for clinical studies. Clearance kinetics from a common subcutaneous web space injection depot in normal volunteers is a convenient way in which to test the validity of dual-labeled material, even if the ultimate purpose is use in a different setting, such as the tumour bed in SLN lymphoscintigraphy.
References
- Chagpar AB, McMasters KM. Sentinel lymph node biopsy for breast cancer: From investigational procedure to standard practice. Expert Rev Anticancer Ther 2004; 4: 903–12
- Eedy DJ. Introducing controversies in dermatology: Sentinel lymph node biopsy for cutaneous melanoma-a useful technique or a waste of time?. Br J Dermatol 2004; 151: 267–8
- Werner JA, Dunne AA, Ramaswamy A. The sentinel node concept in head and neck cancer: Solution for the controversies in the N0 neck?. Head Neck 2004; 26: 603–11
- Terada KY, Shimizu DM, Wong JH. Sentinel node dissection and ultrastaging in squamous cell cancer of the vulva. Gynecol Oncol 2000; 76: 40–4
- Faries MB, Bleicher RJ, Ye X, Essner R, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for primary and metastatic pulmonary malignant neoplasms. Arch Surg 2004; 139: 870–6
- Braat AE, Oosterhuis JW, Moll FC. Successful sentinel node identification in colon carcinoma using Patent Blue V. Eur J Surg Oncol 2004; 30: 633–7
- Wagner JD, Schauwecker D, Davidson D, Coleman JJ, Saxman S, Hutchins G, et al. Prospective study of fluorodeoxyglucose-positron emission tomography imaging of lymph node basins in melanoma patients undergoing sentinel node biopsy. J Clin Oncol 1999; 17: 1508–15
- Reintgen DS, Cruse CW, Wells K, Berman C, Fenske N, Glass F, et al. The orderly progression of melanoma nodal metastases. Ann Surg 1994; 220: 759–67
- Statius Muller MG, Borgstein PJ, Pijpers R, van Leeuwen PA, van Diest PJ, Gupta A, et al. Reliability of the sentinel node procedure in melanoma patients: Analysis of failures after long-term follow-up. Ann Surg Oncol 2000; 7: 461–8
- Tanis PJ, Nieweg OE, Valdes Olmos RA, Kroon BB. Anatomy and physiology of lymphatic drainage of the breast from the perspective of sentinel node biopsy. J Am Coll Surg 2001; 192: 399–409
- Noguchi M. Current controversies concerning sentinel lymph node biopsy for breast cancer. Breast Cancer Res Treat 2004; 84: 261–71
- Celliers L, Mann GB. Alternative sites of injection for sentinel lymph node biopsy in breast cancer. ANZ J Surg 2003; 73: 600–4
- Chagpar A, Martin RC, Chao C, Wong SL, Edwards MJ, Tuttle T, et al. Validation of subareolar and periareolar injection techniques for breast sentinel lymph node biopsy. Arch Surg 2004; 139: 614–8
- Pain SJ, Nicholas RS, Barber RW, Ballinger JR, Purushotham AD, Mortimer PS, et al. Quantification of lymphatic function for investigation of lymphoedema: Depot clearance and rate of appearance of soluble macromolecules in blood. J Nucl Med 2002; 43: 318–24
- Pain SJ, Barber RW, Ballinger JR, Solanki CK, O'Mahony S, Mortimer PS, et al. Side-to-side symmetry of radioprotein transfer from tissue space to systemic vasculature following subcutaneous injection in normal subjects and patients with breast cancer. Eur J Nucl Med Mol Imaging 2003; 30: 657–61
- Pain SJ, Barber RW, Ballinger JR, Solanki CK, Mortimer PS, Purushotham AD, et al. Transport of radiolabeled immunoglobulin injected into the web spaces of the hands of normal subjects and patients with breast cancer-related lymphedema. J Vasc Res 2004; 41: 183–92
- Pain SJ, Barber RW, Ballinger JR, Solanki CK, Mortimer PS, Purushotham AD, et al. Local vascular access of radioprotein injected subcutaneously in normal subjects and patients with breast cancer-related lymphedema. J Nucl Med 2004; 45: 789–96
- Mitterhauser M, Wadsak W, Mien LK, Eidherr H, Roka S, Zettinig G, et al. The labeling of Nanocoll® with [111In] for dual-isotope scanning. Appl Radiat Isot 2003; 59: 337–42
- Ergun EL, Ercan MT, Asansu A, Unsal IS. Evaluation of 99mTc-HIG as a lymphoscintigraphic agent in rabbits. Nucl Med Commun 1998; 19: 665–70
- Svensson W, Glass DN, Bradley D, Peters AM. Measurement of lymphatic function with Tc-99m labeled polyclonal immunoglobulin. Eur J Nucl Med 1999; 26: 504–10
- Hollander W, Reilly P, Burrows BA. Lymphatic flow in human subjects as indicated by the disappearance of 131I labeled albumin from the subcutaneous tissue. J Clin Invest 1961; 40: 222–3
- Fowler JC, Solanki CK, Barber RW, Swift EA, Guenther I, Ballinger JR, et al. Measurement of the extraction fractions of nanocolloid and polyclonal immunoglobulin by axillary lymph nodes in patients with breast cancer. Nucl Med Commun 2004; 25: 935–40