Abstract
The aim of this retrospective study was to compare the value of FDG-PET with conventional imaging in patients with residual disease or suspected relapse in Hodgkin's lymphoma (HL). We reviewed the records of all patients with HL who were referred for FDG-PET at our PET centre between April 2002 and August 2004. Thirty-four FDG-PET scans performed on 26 patients were included in the study. Referrals were based on either the presence of a residual mass on computed tomography (CT) (n = 13) or suspicion of relapse (n = 21). We found one false negative and one false positive FDG-PET scan. The high positive predictive value of FDG-PET in the residual group and the high negative predictive value in the relapse group strongly indicate that FDG-PET has an important role to play in the management of HL.
The cure rate of patients with Hodgkin's lymphoma (HL) has dramatically improved during recent decades. The 10-year overall survival in early stages is currently about 95%. In intermediate stages 95% of the patients achieve complete remission, but 15% of these patients suffer an early relapse. In advanced stages the 5-year freedom from treatment-failure rate is about 85% Citation[1]. In two-thirds of the HL patients the mediastinum is involved at diagnosis, and in one-third of these cases mediastinal involvement is considered bulky (mediastinal:thoracic ratio ≥0.33). In patients with bulky disease (lymph node > 10 cm in diameter or bulky mediastinum) a residual mass usually remains after completed therapy. It is essential to distinguish between active lymphoma tissue and fibroid remnants. If vital tumour tissue remains, further therapy should be started as soon as possible. On the other hand, if no active disease is present the patient may be spared additional morbidity from unnecessary treatment. To date, no feasible tools sensitive enough to make the distinction between active residual disease and fibroid remnants have been available.
Positron emission tomography (PET) with 18F-2-fluoro-2-deoxy-D-glucose (FDG) visualizes FDG metabolism in vivo. Malignant tumours show elevated glucose metabolism compared with normal tissue. FDG-PET is thus able to visualize tumour activity. HL is FDG avid and several studies have shown the usefulness of FDG-PET in visualizing tumor involvement in HL Citation[2–4]. Visual evaluation of the PET scan is almost always sufficient in lymphomas, and quantitative methods have little to add Citation[5]. Quantification of metabolism (MR; metabolic rate) may offer additional information in therapy monitoring when sequential PET studies are performed during cytotoxic treatment. This is an area currently undergoing intense investigation in HL and other tumour types.
The purpose of this retrospective study was to compare the value of FDG-PET with conventional imaging in HL patients with residual tumour or suspected relapse.
Patients and methods
Between April 2002 and August 2004, 30 patients with HL were referred for FDG-PET examination at the Department of Oncology at Lund University Hospital, Sweden, because of the presence of a residual tumour after therapy or because of a suspected relapse. The records of all 30 patients were reviewed and 26 were included in the study. The four patients not included were either lost to follow-up (n = 2), had not followed the PET scan preparation instructions (i.e. had not fasted) (n = 1) or had been re-evaluated as having non-Hodgkin's lymphoma (NHL) (n = 1). All patients had biopsy-proven HL according to the WHO classification Citation[6]. Patients were divided into two groups based on the reason for referral for FDG-PET: residual tumour or suspected relapse. During the study period five patients underwent PET scans for both residual tumour and suspected relapse, and thus occur in both groups. The study was approved by the local ethics committee.
Referral because of residual tumour
In this group 13 patients (eight females and five males) underwent 14 FDG-PET scans (one patient underwent two scans). All patients were found to have residual mass on computed tomography (CT). The median age at the first FDG-PET scan was 29 (17 – 54) years, and the median time interval between most recent treatment and PET scan was 4 (0.5 – 12) months. Patient characteristics are listed in . The mean follow-up time after FDG-PET was 16 (9 – 33) months.
Table I. Patient characteristics, residual group (n = 13).
Referral because of suspected relapse
Eighteen patients (seven females and 11 males) underwent 20 PET scans (two patients underwent two scans). All patients were in complete remission (CR) following primary or salvage therapy. The suspicion of relapse was based on symptoms reported by patients, findings at physical examination and/or, inconclusive findings at CT. For details, see . The median age at the first FDG-PET scan was 29 (8 – 70) years and the median time interval between most recent treatment and FDG-PET was 6.5 (1 – 30) months. The mean follow-up time after FDG-PET was 12 (7 – 27) months. Relapse was defined as ‘histopathologic evidence of relapse, obtained by either biopsy or fine-needle aspiration at the clinician's discretion’.
Table II. Reason for referral to PET, relapse group (n = 20).
PET
PET was performed in a dedicated whole-body PET scanner (Scanditronix 2048). Patients fasted for at least four hours before i.v. injection of 18F-FDG with an average activity of 342 (90 – 615) MBq. A blood sample was taken from each patient before the injection for evaluation of the glucose level. No patient in this study was hyperglycaemic. One patient had insulin-dependent diabetes, but through restricted diet and insulin medication normoglycaemia was obtained and the PET scan was of good quality. Patients rested 60 – 90 minutes following the FDG-injection before scanning. When abdominally located lesions were suspected diuretics (furosemide, 20 mg i.v.) and water (7 – 10 dl orally) were given immediately following FDG to achieve forced excretion of FDG through the urinary system. PET images from the upper part of the thighs to the base of the scull were obtained on a Scanditronix 2048 whole body scanner, with a spatial resolution of 6.5 mm in the centre of the field of view. The non-attenuation corrected images were reconstructed using an iterative reconstruction algorithm. All images were visually evaluated by two experienced investigators with access to clinical data. Any focus of elevated FDG metabolism not located in areas of normal FDG uptake or where the clinical data did not suggest the presence of non-malignant hypermetabolic lesions (i.e. inflammatory or infectious foci) were interpreted as positive for viable HL.
Conventional imaging
All patients underwent conventional imaging within two weeks before or after the FDG-PET scan. CT was performed in all but one case, in which magnetic resonance imaging (MRI) was performed. All patients referred for FDG-PET in the residual group had a remaining mass on CT. In the relapse group a negative CT scan was defined as the absence of a malignant or undetermined lesion and a positive CT scan was defined as the presence of either a mass of uncertain value or a mass characterized as malignant by the radiologist.
Results
Patients with residual tumour
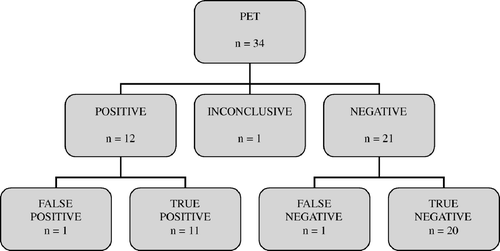
Of the 14 FDG-PET scans performed in this group, three scans were positive for residual disease and 11 negative. The presence of active lymphoma tissue was confirmed by fine-needle aspiration in all three patients. One of the 11 patients with negative FDG-PET scans relapsed after six months. When we reviewed the images retrospectively, a small area of increased FDG metabolism could be detected at the site of relapse in the mediastinum. The PET scan was thus recorded as false negative. The results are presented in and .
Table III. Results.
Relapse group
In this group 20 FDG-PET scans were performed and eight relapses were verified by either biopsy or fine-needle aspiration depending on the clinical situation. Only five of the patients with verified relapse had reported any symptoms (patients 6, 12, 14, 15 and 18 in ). Nine FDG-PET scans were positive, i.e. showed hypermetabolic lesions. All eight relapses were detected. A false positive FDG-PET was recorded in a patient where further investigation, including biopsy, could not confirm relapse. Repeated FDG-PET six weeks later was negative and the patient was still in complete remission eight months after the first FDG-PET scan. Ten FDG-PET scans were negative and all of them were confirmed to be true negative by a mean event-free follow-up time of 14 (7 – 27) months. One FDG-PET scan showed an area of slightly elevated metabolism of unclear delineation in the abdomen of a patient with previous abdominal involvement. The result was recorded as inconclusive. Follow-up has been event-free for eight months. The results are presented in and .
Discussion
In the present study we have shown that FDG-PET improves the diagnostic accuracy in patients with HL presenting a residual tumour or suspicion of relapse. Of a total of 34 FDG-PET scans we found 12 to be positive for metabolically active disease, 21 to be negative, and inconclusive in one case. Only one false negative and one false positive scan were recorded (). With conventional imaging (CT and, in one case, MRI) inconclusive results were common, i.e. 12 of 34 cases. Besides demonstrating that FDG-PET is able to correctly identify viable lymphoma tissue to a greater extent than conventional imaging, we have also shown that a negative FDG-PET result reaches a high level of reliability. The positive predictive value and negative predictive value for FDG-PET in the evaluation of residual mass were 100% and 91%, and in the detection of suspected relapse, 89% and 100%.
Recently, a number of studies have indicated that FDG-PET can make a valuable contribution in the management of HL at initial staging in detecting additional sites of involvement Citation[4], Citation[7], for early response assessment Citation[8], Citation[9], in post-treatment evaluation Citation[10–12] and in the detection of relapse Citation[3]. FDG-PET has also been shown to be superior to CT and MRI in detecting residual disease. This was recently confirmed in a review by the Health Technology Assessment (HTA) in England Citation[13]. In another recent review of the literature the authors concluded that there is strong evidence that FDG-PET detects more disease sites and involved organs than conventional imaging modalities and has a large influence on staging Citation[14]. See for a comparison with recently published studies.
Table IV. Comparison with recently published studies.
Residual disease
One of the clinical challenges in the management of HL is to discriminate between active residual disease and non-malignant tissue after the completion of standard therapy. This is a common problem which occurs in the majority of HL patients; although no solid figures on this matter have been reported to the best of our knowledge. Overtreatment implies the risk of further severe toxicity and side effects in an already cured patient, while not providing further therapy to a patient with active residual disease means, of course, the risk of not curing the patient. Until recently, the discrimination between active residual disease and non-malignant tissue has not been possible without extensive examination, including high-risk procedures such as radical surgery. And thus, the watch-and-wait policy has usually been the recommended path, with repeated examinations at predetermined time intervals. Several studies have been published on the role of FDG-PET in the evaluation of residual tumour masses, see . Many of these reports are based on a mixed population of lymphomas, both HL and NHL. This, of course, limits the conclusions that can be drawn from the data since a residual mass in NHL represents viable tumour far more often than in HL Citation[15].
Weihrauch and co-workers presented results from a study performed exclusively on HL patients Citation[10]. They prospectively included 28 patients with HL and a residual mass visible on CT or, in one case, MRI after primary or salvage therapy. All patients underwent FDG-PET and were monitored for at least one year after the PET-study. For FDG-PET, the negative predictive value was 95% and the positive predictive value was 60% at 1-year follow-up. The authors concluded that the low positive predictive value could be explained by the equal risk of relapse and complete clinical remission when a residual mass is visible on CT. de Wit and colleagues have also reported results of a prospective study on 37 patients with a residual mass after treatment of HL who underwent 50 PET scans. In their study FDG-PET showed a sensitivity of 91% and specificity of 69% compared with 72% and 21% for conventional CT scans Citation[11]. Our study supports these findings, especially the high negative predictive value and the high values of sensitivity and specificity for FDG-PET.
Relapse
When relapse is suspected it is important to verify or reject the suspicion. One would expect early recognition of a relapse to improve outcome, but this has not yet been proven. A few studies employing FDG-PET in recurrent HL have been published (see ), but there is no worldwide consensus concerning the follow-up procedures after completion of treatment. Torrey and colleagues Citation[16], who retrospectively examined follow-up procedures of 709 patients treated for HL between 1969 and 1994, found that the rate of relapse detection was highest when using a combination of patient history and physical examination, and was by far the most cost-effective method of follow-up. The same conclusion was drawn by Radford and co-workers Citation[17]. In their retrospective study of 210 HL patients they found that recurrence was usually detected as the result of the investigation of symptoms, rather than by routine screening of asymptomatic patients. On the other hand, Jerusalem et al. described a study of 36 patients with HL who underwent PET at the end of treatment and then every 4 – 6 months during follow-up for 2 – 3 years Citation[3]. Although the study showed a high proportion of false positive findings, PET correctly identified every relapse, whereas neither clinical examination, laboratory findings nor CT identified any relapse at all. The investigators concluded that PET can be positive up to nine months before histological confirmation of an asymptomatic relapse, and that FDG-PET should be used as the follow-up method of choice at an interval of 6 – 8 months. Our results confirm that FDG-PET is superior to CT when relapse is suspected. In our study nine false positive CT results and one false negative CT result were recorded in the relapse group, showing a clinical benefit with FDG-PET as compared to conventional imaging modalities.
Shortcomings of PET
A number of studies have addressed the shortcomings of FDG-PET in HL. One obvious limitation is the size of the lesion. Very small tumours, less than 6 – 7 mm, remain undetected in currently used, dedicated PET systems, the problem being more obvious in the abdomen than in the thorax. This is partly due to the underestimation of the metabolic activity in small lesions. Furthermore, the level of hypermetabolism is of importance. Low-grade hypermetabolism is more difficult to identify than a highly metabolically active lesion, since the PET result depends on the relative difference in glucose metabolism between normal and malignant tissue. This may be illustrated by the one false negative FDG-PET scan in our study. It was recorded in the residual group in a patient who was in complete response, with a residual mass considered benign on CT and a negative FDG-PET scan, after primary treatment. A relapse was diagnosed six months later at the location of the residual mass and the FDG-PET scan was positive at this time. This demonstrates the known inability of PET to detect very limited disease.
Another problem associated with FDG-PET in HL is the often high rate of false positive findings in areas other than known locations of malignant hypermetabolism, such as thymic hyperplasia, brown fat and physiological uptake in paravertebral and supraclavicular regions, which is often seen in young subjects and thus often in a population of HL patients Citation[18], Citation[19]. Furthermore, reactive hypermetabolism in inflammatory, infectious and granulomatous lesions can mimic malignant FDG-metabolism Citation[20] which may cause false-positive findings. The false positive scan in our study was recorded in the relapse group in an 8-year-old patient five months after the completion of radiotherapy. CT was performed as part of a routine follow-up programme and was inconclusive, which was the reason the patient was referred for a FDG-PET scan. This showed a distinct hypermetabolic lesion in the thorax. A new PET scan six weeks later was completely normal but CT was still inconclusive. The hypermetabolic focus on the first PET scan was thought to have represented reactive changes following radiotherapy. This false positive result could probably not have been avoided, even if we had added a quantitative or semi-quantitative method to the procedure, although this is purely speculative. The most widespread quantification method, SUV (standardized uptake value), is usually not helpful under these circumstances and no other method has been validated as of yet. Attenuation correction was not carried out in our PET studies and we do not believe that this would have improved the quality of the interpretations, since the procedure also introduces noise into the pictures. It has previously been demonstrated that attenuation correction does not improve diagnostic accuracy in HL Citation[21]. With coming improvements in PET systems leading to the detection of smaller lesions, and the more widespread use of fused images in combined PET-CT scanners, PET studies will without doubt become more reliable in the near future.
Another drawback is the cost and availability of PET equipment. In a global perspective, PET is not available to the vast majority of patients on a global scale and the distribution of PET centres is extremely uneven. Bearing this in mind it is crucial to carefully evaluate the role of PET in the management of clinical problems to identify situations where the benefit surpasses the costs, before it is adopted in routine use.
Conclusions
In conclusion, the present study clearly confirms, despite the small number of subjects, that FDG-PET can play an important role in HL when a residual mass is present and can contribute to the diagnostic procedure when a relapse is suspected. FDG-PET plays an increasingly important role in the management of HL. Ongoing studies will provide us with more specific recommendations concerning when to use FDG-PET. We predict that the main focus will shift from primary staging to therapy monitoring and evaluation of residual disease in the near future. Based on the present level of evidence we suggest that FDG-PET be performed when remaining tissue is detected at a known tumour location after treatment of HL. We also recommend that the use of FDG-PET be seriously considered when recurrence of HL is suspected since FDG-PET can provide additional important information with a strong negative predictive value.
References
- Diehl V, Thomas RK, Re D. Part II: Hodgkin's lymphoma–diagnosis and treatment. Lancet Oncol 2004; 5: 19–26
- Hueltenschmidt B, Sautter-Bihl ML, Lang O, Maul FD, Fischer J, Mergenthaler HG, et al. Whole body positron emission tomography in the treatment of Hodgkin disease. Cancer 2001; 91: 302–10
- Jerusalem G, Beguin Y, Fassotte MF, Belhocine T, Hustinx R, Rigo P, et al. Early detection of relapse by whole-body positron emission tomography in the follow-up of patients with Hodgkin's disease. Ann Oncol 2003; 14: 123–30
- Weihrauch MR, Re D, Bischoff S, Dietlein M, Scheidhauer K, Krug B, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose for initial staging of patients with Hodgkin's disease. Ann Hematol 2002; 81: 20–5
- Weber WA. Use of PET for monitoring cancer therapy and for predicting outcome. J Nucl Med 2005; 46: 983–95
- Jaffe ES HNL, Stein H, Vardiman JW, editors. World Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001.
- Munker R, Glass J, Griffeth LK, Sattar T, Zamani R, Heldmann M, et al. Contribution of PET imaging to the initial staging and prognosis of patients with Hodgkin's disease. Ann Oncol 2004; 15: 1699–704
- Kostakoglu L, Coleman M, Leonard JP, Kuji I, Zoe H, Goldsmith SJ. PET predicts prognosis after 1 cycle of chemotherapy in aggressive lymphoma and Hodgkin's disease. J Nucl Med 2002; 43: 1018–27
- Hutchings M, Loft A, Hansen MT, Pedersen LM, Buhl T, Jurlander J, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin Lymphoma. Blood 2006; 107: 52–9
- Weihrauch MR, Re D, Scheidhauer K, Ansen S, Dietlein M, Bischoff S, et al. Thoracic positron emission tomography using 18F-fluorodeoxyglucose for the evaluation of residual mediastinal Hodgkin disease. Blood 2001; 98: 2930–4
- de Wit M, Bohuslavizki KH, Buchert R, Bumann D, Clausen M, Hossfeld DK. 18FDG-PET following treatment as valid predictor for disease-free survival in Hodgkin's lymphoma. Ann Oncol 2001; 12: 29–37
- Guay C., Lépine M, Verreault J, Bénard F. Prognostic value of PET using 18F-FDG in Hodgkin′s disease for posttreatment evaluation. J Nucl Med 2003; 44: 1225–31
- Facey K, Bradbury I, Laking A, Payne E. “Ultra rapid review. Positron Emission Tomography (PET) Imaging in Cancer Management” HTA July 2004.
- Hutchings M, Eigtved AI, Specht L. FDG-PET in the clinical management of Hodgkin lymphoma. Crit Rev Oncol Hematol 2004; 52: 19–32
- Jerusalem G, Beguin Y, Fassotte MF, Najjar F, Paulus P, Rigo P, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose for posttreatment evaluation in Hodgkin's disease and non-Hodgkin's lymphoma has higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood 1999; 94: 429–33
- Torrey MJ, Poen JC, Hoppe RT. Detection of relapse in early-stage Hodgkin's disease: Role of routine follow-up studies. J Clin Oncol, vol 15, No 3 (March), 1997:1123–1130.
- Radford JA, Eardley A, Woodman C, Crowther D. Follow up policy after treatment for Hodgkin's disease: Too many clinic visits and routine tests? A review of hospital records. BMJ 1997; 314: 343–6
- Kostakoglu L, Goldsmith SJ. Fluorine-18 fluorodeoxyglucose positron emission tomography in the staging and follow-up of lymphoma: Is it time to shift gears?. Eur J Nucl Med 2000; 27: 1564–78
- Dobert N, Menzel C, Hamscho N, Wordehoff W, Kranert WT, Grunwald F. Atypical thoracic and supraclavicular FDG-uptake in patients with Hodgkin's and non-Hodgkin's lymphoma. Q J Nucl Med Mol Imaging 2004; Vol 48, No 1:33–38.
- de Hemricourt E, De Boeck K, Hilte F, Abib A, Kockx M, Vandevivere J, et al. Sarcoidosis and sarcoid-like reaction following Hodgkin's disease. Report of two cases. Mol Imaging Biol 2003; 5: 15–9
- Kotzerke J, Guhlmann A, Moog F, Frickhofen N, Reske SN. Role of attenuation correction for fluorine-18 fluorodeoxyglucose positron emission tomography in the primary staging of malignant lymphoma. Eur J Nucl Med 1999; 26: 31–8
- Mikhaeel NG, Timothy AR, Hain SF, O'Doherty MJ. 18-FDG-PET for the assessment of residual masses on CT following treatment of lymphomas. Ann Oncol 2000; 11(Suppl 1)147–50
- Dittmann H, Sokler M, Kollmannsberger C, Dohmen BM, Baumann C, Kopp A, et al. Comparison of 18FDG-PET with CT scans in the evaluation of patients with residual and recurrent Hodgkin's lymphoma. Oncol Rep 2001; 8: 1393–9
- Naumann R, Vaic A, Beuthien-Baumann B, Bredow J, Kropp J, Kittner T, et al. Prognostic value of positron emission tomography in the evaluation of post-treatment residual mass in patients with Hodgkin's disease and non-Hodgkin's lymphoma. Br J Haematol 2001; 115: 793–800
- Zinzani PL, Magagnoli M, Chierichetti F, Zompatori M, Garraffa G, Bendandi M, et al. The role of positron emission tomography (PET) in the management of lymphoma patients. Ann Oncol 1999; 10: 1181–4
- Spaepen K, Stroobants S, Dupont P, Thomas J, Vandenberghe P, Balzarini J, et al. Can positron emission tomography with [(18)F]-fluorodeoxyglucose after first-line treatment distinguish Hodgkin's disease patients who need additional therapy from others in whom additional therapy would mean avoidable toxicity?. Br J Haematol 2001; 115: 272–8