To the editor The pro-fibrotic cytokine transforming growth factor β1 (TGFβ1) is implicated in the pathogenesis of radiation fibrosis Citation[1–6], which contributes to late morbidity following radiotherapy. Relatively few laboratories have conducted studies during radiotherapy and none to our knowledge during pelvic radiotherapy. Neither have strict precautions been observed to minimise platelet degradation and supra-physiological TGFβ1 release. Excessive platelet degranulation during standard venepuncture causes immediate release of TGFβ1 and further increments can occur during long-term storage of blood/serum unless samples are frozen at −20°C. These factors hinder reliable inter-comparisons of results from different laboratories.
Here we report a detailed study involving eight patients: five had cervix cancers and received radical radiotherapy (at 1.8 Gy per fraction) and weekly cis-platinum chemotherapy (numbers 2, 4, 5, 7 and 8), while three received pelvic radiotherapy after hysterectomy for endometrial malignancy (numbered 1, 3 and 6). Following informed consent procedures, venous blood was collected without a tourniquet, through a wide-gauge butterfly needle into a pre-chilled CTAD tube. Vacutainers and syringes were not used because of the turbulence (and platelet degradation) they induce. Samples were gently mixed by inversion and placed immediately on ice. Within one hour samples were centrifuged for 30 min at 1200×g at 4oC. One millilitre aliquots of supernatant were stored at −70oC. All plasma samples were assayed for TGFβ1 within six months of collection in order to minimise the increases during long-term storage which have been reported in previous studies and which are thought to result from release of covalently bound TGFβ1 from α-macroglobulin Citation[7]. In order to avoid inclusion of latent TGFβ1 complexes in human plasma, we used the R&D Quantikine ELISA System for human TGFβ1, with pre-treatment of samples with 2.5N acetic acid/10M urea to disrupt a range of TGFβ1 complexes. Each sample was assayed in duplicate at three serial dilutions and the mean and standard error calculated from the resulting six readings.
The baseline plasma TGFβ1 results (see ) were generally lower than the published data relating to lung and breast cancer, which is consistent with our sample collection technique. The standard error values are small. Most remained below 5 ng/ml during treatment, but two patients had levels >10 ng/ml. Close inspection of reveals that there are possible cyclical fluctuations in plasma TGFβ1. Such fluctuations could be due to random variation (or are associated with the assay technique), rather than being a result of ordered homeostatic cyclical activity. Another possibility is that results were heavily influenced by the weekly sampling times: for example, if twice weekly sampling had been used, then further fluctuations and a shorter cyclical period might have been found. One patient (number 5) with recurrent cancer and who had undergone previous radiotherapy and extensive pelvic surgery had a grossly elevated baseline level rising to 29 ng/ml. Fourier analysis of the 48 data points suggests an initial depression and later elevation in plasma TGFβ1 during radiotherapy (patients 1, 3 and 4) and no significant changes in all others. Further studies are required to determine normal temporal variations in plasma TGFβ1, the significance of perturbations during radiotherapy and any possible association with late tissue effects.
Figure 1. Plasma TGFβ1 before and during pelvic radiotherapy in eight patients (mean and SEM of six readings). # 0 indicates the control value. Subsequent samples were taken at weekly intervals while the radiation dose increased by 9 Gy per week at 1.8 Gy per fraction. Patients are numbered 1–8.
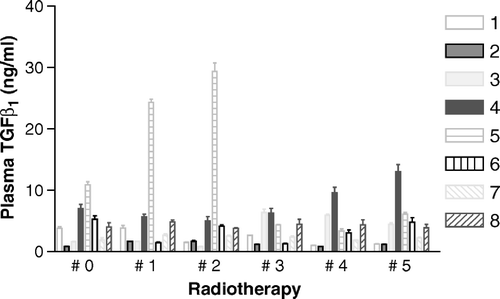
From the limited data available it is not possible to state which week would be the best to determine TGFβ1 responses, although the final fifth week of the study appears to be discriminating in the case of patient 4. The value for this patient is beyond the 95th percentile of all values of those treated for primary rather than recurrent cancers.
Our results for baseline plasma TGFβ1 levels are broadly consistent with previously published data. In various groups of normal subjects these vary from 0.1 ng/ml to over 25 ng/ml, Citation[7] but where steps have been taken to minimise platelet degranulation in healthy subjects the published range is 2.0–12.0 ng/ml. Previous studies in patients with lung cancer have shown mean pre-treatment levels of 11.7 ng/ml, Citation[5] while breast cancer patients show mean pre-treatment levels of approximately 20 ng/ml Citation[4]. Our lower levels, found in previously untreated patients (range 0.81 to 10.97 ng/ml; median 2.83 ng/ml), are likely to reflect the careful precautions taken to minimise platelet degradation.
There is concern that concomitant chemo-radiotherapy using platinum-based chemotherapy might enhance the risk of late normal tissue complications. In this limited study there appears to be no detectable enhanced acute TGFβ1 response in three out of four patients, but one patient did develop higher levels later during concomitant chemo-radiotherapy. The effects of post-surgical healing after hysterectomy do not appear to have produced elevated TGFβ1 levels in the three patients so studied. Follow up was short (range 6 – 20 months, median 17 months) and no patient experienced severe late morbidity in that time.
We recommend that further ‘horizontal’ studies of the variations in plasma TGFβ1 levels with time, using more frequently taken samples (e.g. twice or three times per week), should be performed in normal individuals. Such studies will determine whether or not true background temporal variations exist.
Further research in this area will require the best possible assays in order to determine prospectively – in large numbers of patients-whether elevated levels during (or soon after) radiotherapy correlate with severe late effects.
References
- Anscher MS, Kong F-M, Andrews K, Clough R, Marks LB, Bentel G, et al. Plasma transforming growth factor as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys 1998; 41: 1029–35
- Anscher MS, Marks LB, Shafman TD, Clough R, Huang H, Tisch A, et al. Risk of long-term complications after TGF-beta1-guided very-high-dose thoracic radiotherapy. Int J Radiat Oncol Biol Phys 2003; 56: 988–95
- Chen Y, Williams J, Ding I, Hernady E, Liu W, Smudzin T, et al. Radiation pneumonitis and early circulatory cytokine markers. Semin Radiat Oncol 2002; (Suppl1): 26–33
- Kong F-M, Anscher MS, Murase T, Abbott BD, Iglehart D, Jirtle RL. Elevated transforming growth factor-β1 levels in breast cancer patients decrease after surgical removal of the tumor. Ann Surg 1995; 222: 155–62
- Kong F, Jirtle RL, Huang DH, Clough RW, Anscher MS. Plasma transforming growth factor-β1 level before radiotherapy correlates with long term outcome of patients with lung carcinoma. Cancer 1999; 86: 1712–9
- Martin M, Lefaix J, Delanian S. TGFβ1 and radiation fibrosis: A master switch and a specific therapeutic target?. Int J Radiat Oncol Biol Phys 2000; 47: 277–90
- Grainger DJ, Mosedale DE, Metcalfe JC. TGFβ in blood: A complex problem. Cytokine & Growth Factor Reviews 2000; 11: 133–45