Abstract
The aim of this study is to determinate incidence and risk factors for loco regional failure (LRR) (breast, supraclavicular, axillary and internal mammary nodes) and indications for nodal irradiation. From January 1980 to December 2001, 4185 patients with T1–T2 breast cancer were treated with conservative surgery and whole breast radiotherapy without nodal irradiation at the University of Florence. The median age was 55 years (range 19–86). All patients were followed for a median of eight years (range 3 months to 20 years). Multivariate analysis showed as independent prognostic factors for isolated nodal relapse (NR) the presence of more than three positive lymph nodes (PAN) (p = 0.001), angiolymphatic invasion (p = 0.002) and pT2 (p = 0.02). However, only 4.8% of patients with more than three PAN developed NR as the only site of recurrence. Having one to three PAN was not associated with an increased risk of NR. We believe that it is not necessary to prescribe nodal irradiation to patients with negative or one to three PAN. Regarding patients with more than three PAN, the number of isolated NR is also small to routinely justify a node irradiation.
The overall survival of early breast cancer patients treated by conservative surgery and radiation has been well documented Citation[1–3] while the patterns of failure and outcome of patients experiencing a local regional recurrence (LRR) (breast and supraclavicular, axillar and internal mammary nodes) are less well known. Previous studies have suggested that the incidence of LRR (breast, supraclavicular, axillar and internal mammary nodes) after lumpectomy and adjuvant radiotherapy in early breast cancer ranges between 4–20% and it is related to patient's age, status of surgical margin, presence of perivascular invasion and intraductal in situ carcinoma and lymph nodes status Citation[4–8]. The optimal treatment of regional nodes in the setting of breast-conserving surgery and radiation after early-breast cancer is unclear.
The aim of the present analysis is to determinate the incidence and risk factors for loco regional failure and to evaluate if a sub-group of patients should receive nodal irradiation.
Methods and materials
From January 1980 to December 2001, 4185 patients with pT1–T2 breast cancer underwent radiotherapy treatment at the University of Florence. In the current analysis we included patients without clinical and radiographic evidence of local or distant recurrence after surgery at the time of the first evaluation in the radiotherapy unit. None of the patients had prior malignant disease. The median age of the patient population was 55 years (range 19–86). No patients were lost at follow-up. All patients were followed for a median of eight years (range 3 months to 20 years).
The main characteristics of the patients are reported in . Wide excision was performed in 919 patients and 3266 patients underwent quadrantectomy. Whenever possible patients with positive surgical margins underwent reexcision, however, 621 patients underwent radiotherapy with positive surgical margins. Axillary dissection was performed in 3889 patients with a median number of 16 nodes removed. Positive axillary nodes were found in 1215 patients (29%). Sentinel lymph node biopsy was performed only on 170 (4%) patients and axillary dissection was performed in 100 (2.3%) patients.
Table I. Characteristics of the 4185 patients, University of Florence, 1980–2001.
According to the protocol followed in our Institute, radiotherapy was not given to supraclavicular fossa also for patients with positive axillary nodes. All patients received radiotherapy treatment (RT) only to whole breast.
All patients were treated with external beam radiotherapy to the whole breast using tangential fields with 6 MV photons. The mean dose delivered was 50 Gy, 2 Gy daily fraction (range 46–52). The tumour bed boost was administrated by electrons. At the discretion of the radiation oncologist the total boost dose (2 Gy daily fraction) ranged between 6 and 10 Gy for patients with negative surgical margins and between 14 and 16 Gy for patients with positive margins. In 23.3% of patients (972 of 4185) no boost was administered.
Chemotherapy was recommended in 924 patients (21.8%). Of those 20% received anthracycline based chemotherapy: 70% of these received four courses of epidoxorubicin (100 mg/m2) followed by four courses of IV CMF (cyclophosfamide 600 mg/m2, metotrexate 40 mg/m2 and 5-fluorouracil 600 mg/m2) and 30% were treated with six courses of FEC chemotherapy (5-fluorouracil 500 mg/m2, epidoxorubicin 75 mg/m2, cyclophosfamide 500 mg/m2). Sixty-five percent of the patients received six courses of IV CMF (cyclophosfamide 600 mg/m2, metotrexate 40 mg/m2 and 5-fluorouracil 600 mg/m2) and 15% other types of chemotherapy. There were 133 patients (3.1%) treated with chemotherapy in different institutions. Tamoxifen was prescribed in 1504 patients (36%) whereas 2241 patients (63%) received no hormonal treatment. No such information is available for 40 patients (1%). Tamoxifen was not prescibed from 1980 to 1986. From 1987 to 1990 only 3.45% of patients (50 of 1504) were treated with tamoxifen.
Statistical analysis
For statistical analysis we considered as true LRR when failure developed as first site of recurrence and when loco-regional failure was followed by distant metastases not before six months. We chose this approach because this group of LRR could be a door from which cancer could spread elsewhere.
We considered as LRR any reappearence of tumoral disease in the breast, supraclavicular fossa, axilla and internal mammary chain. For LRR the follow-up routine procedure required clinical examination every six months, mammography and chest X-ray every year and in cases of clinical or radiological suspect breast or supraclavicular recurrence ultrasound and or chest CT.
Locoregional recurrence rate was analysed using the Kaplan Meier method (Statistica software) Citation[9]. The actuarial probabilities of LRR for the different subgroups were then compared (“internal” comparison). The log-rank test Citation[10] and the Cox model for logistic regression Citation[11] were applied, when appropriate, to define the statistical significance of observed differences. The variables included in the Cox model for multivariate analysis were those judged to be of clinical significance, in addition to those linked with statistically significant difference, when submitted to univariate analysis (log-rank test).
Results
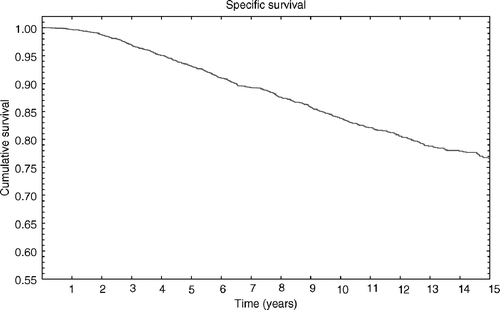
At the time of analysis 12.5% of patients (526/4185) had died of cancer, 4.2% (176/4185) had died of other causes and 83.9% (3512/4185) were still alive. Actuarial cause specific survival for the entire group was 96.9% (±0.2% SE), 93.1% (±0.4% SE), and 83.7 (±0.6% SE) at 3, 5 and 10 years, respectively ().
Figure 1. Cause specific survival for the entire group was 96.9% (±0.2%SE), 93.1% (±0.4%SE) and 83.7 (±0.6%SE) at 3, 5 and 10 respectively.
With a median time of all locoregional relapses (LRR) of 3.9 years (range 6 months–19 years), 224 patients of 4185 (5.3%) had loco-regional failure. With respect to nodal relapses (NR, any relapse in axilla, internal mammary chain or supraclavicular fossa) the median time was lower (2.7 years). Loco-regional relapse was the only failure present in 224 patients. Considering the different sites of LRR we found that the most common site was the breast (166/4185; 3.9%), that NR occurred in 1.3% (58/4185) of patients and supraclavicular relapse (SCR) in 0.7% (33/4185) ().
Table II. Site of recurrence in the 224 patients out of the 4185 developing loco-regional recurrence.
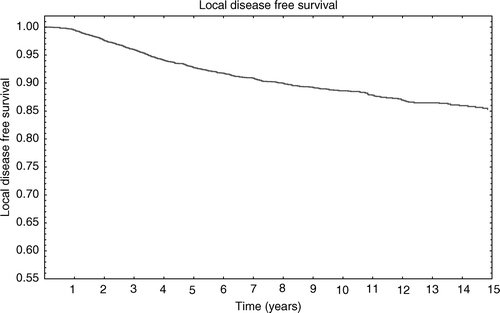
The 3, 5 and 10-year actuarial rate for any LRR were 2.3% (±0.2%SE), 4.3% (±0.4%SE) and 7.4% (±0.5%SE), respectively (). The 3, 5 and 10 years actuarial recurrence rates for SCR were 0.6% (±0.1%SE), 0.9% (±0.2%SE) and 1% (±0.1%SE), respectively, and for NR they were 1.0% (±0.1%SE), 2.0% (±0.2%SE) and 2.0% (±0.2%SE), respectively. Patients aged less than 40 years at diagnosis appear to have a higher LRR rate (14.4%; 49/339) compared to patients aged between 40–49 (8.2%, 83/1006), 50–59 (4.2%; 56/1310) 60–69 (2.8%; 31/1101) 70–79 (1.2%; 5/400) and patients aged more than 80 (0%; 0/29) (p = 0.00001).
Figure 2. The 3, 5 and 10 years actuarial rate for any locoregional recurrence was 2.3% (±0.2%SE), 4.3% (±0.4%SE) and 7.4% (±0.5%SE) respectively.
Analysing the risk of relapse in non-irradiated sites for women under 50 years, who seem to be at higher risk of developing NR, we found that in this subgroup the NR rate was still very low (1.7%; 23/1345). (). LRR rates for pathological tumour size were 4% (110/2779) and 9.1% (114/1406) for pT1 and pT2 tumours respectively (p = 0.0002). NR rates for pT1 and pT2 were 0.8% (25/2779) and 2.3% (33/1406), respectively (p = 0.04). SCR rates for pT1 and pT2 were 0.5% (14/2779) and 1.3% (19/1406), respectively (p = 0.02).
Table III. Correlation between age and LRR.
Analysing LRR by axillary lymph-nodes involvement we observed no significant difference for patients with negative nodes and patients with less than three positive axillary nodes (p = 0.3). However a significant difference was found between patients with negative axillary nodes and patients with more than three positive axillary nodes (p = 0.02) or between patients with one to three positive nodes and more than three (p = 0.05) as shown in .
Figure 3. Local disease free survival by axillary status. 1 = negative axillary nodes; 2 = 1-3 positive axillary nodes; 3 ≥ 3 axillary nodes.
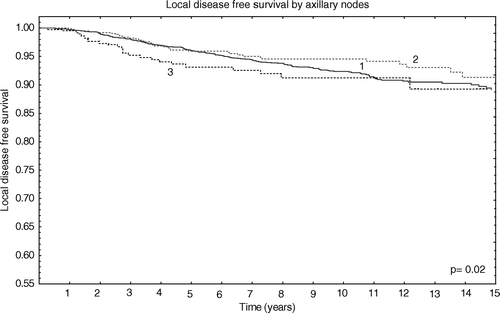
We found a SCR in 12 women of 2870 (0.4%) patients with negative axillary nodes compared with eight women with 1–3 positive axillary lymph nodes (PAN) of 823 (0.9%, p = 0.9) and 13 of 392 (3.3%) women with more than three PAN (p = 0.01) (eight had more than three positive nodes but less than ten; five had more than ten positive axillary nodes). NR was found in 0.8% (24/2870) patients with negative axillary nodes, in 1.7% (14/823) of patients with one to three positive nodes and in 4.8% (19/392) of patients with more than three positive nodes (p = 0.007).
We analyzed the rate of LRR according to the number of removed axillary nodes and we found that it was 3.5% (26/760) for patients with less than ten nodes removed, 5.5% (115/2074) for patients with 10 to 20 nodes removed and 5.5% (68/1251) for patients with more than 20 nodes removed (p = 0.53). We found that this result was neither statistically significant for NR (p = 0.3) nor for SCR (p = 0.9). We did not find any statistical significance for LRR, NR and SCR for the different systemic adjuvant therapies (chemotherapy and hormonal therapy).
Patients with positive angiolymphatic invasion had 6% (29 cases of 485) of LRR versus 3% (61 cases of 2068) for those with negative angiolymphatic invasion (p = 0.001). In 1632 patients the angiolymphatic status was unknown. Considering positive angiolymphatic invasion in relation to positive axillary lymph nodes we did not achieve statistical significance for NR in patients with positive lymph nodes (p = 0.6). However the result was statistically significant (p = 0.0001) in patients with negative lymph nodes.
Univariate analysis of histological grade demonstrated poorer prognosis (p = 0.002) of high grade in comparison to intermediate and low-grade tumours with LRR rates of 3.1% (24/754), 1.8% (14/775) and 1.2% (7/575) respectively. No statistical difference between intermediate and low grade (p = 0.7) was noticed. Tumour grading of 2081 patients was unavailable. However for NR and SCR the histological grade was not found to be statistically significant (p = 0.19).
Other prognostic factors -such as the site of tumor, the extracapsular extention, the multifocality and the different histotypes- did not show any statistical significance at the univariate analysis neither for LRR nor for NR and SCR. Moreover, we reanalaysed the significance of each prognostic factor only in the sub group of patients with 1 to 3 positive axillary nodes. The results are shown in .
Table IV. Crude and actuarial results for each prognostic factor only in the subgroup of patients with 1 to 3 positive axillary nodes.
In the multivariate analysis for LRR G3 tumor (p = 0.01), age at presentation (p = 0.001), more than three positive lymph nodes (p = 0.004), pT2 (p = 0.001) and angiolymphatic invasion (p = 0.02) were statistically significant parameters. In the multivariate analysis for NR only pT2 (p = 0.02), angiolymphatic invasion (p = 0.002) and more than three positive lymph nodes (p = 0.001) were statistically significant ( and ).
Table V. Multivariate analysis for LRR.
Table VI. Multivariate analysis for NR.
Discussion
According to the protocol followed in our institution, we irradiate patients after conserving surgery with tangential beams to the whole breast only, independently of the numbers of PAN.
We have chosen this therapeutic approach due to two considerations:
for patients with less than three PAN the low incidence of LRR does not justify regional nodal treatment;
for patients with more than three PAN the main problem is the risk of distant metastases.
We have considered for this analysis only isolated LRR and NR or LRR and NR followed by distant metastasis after at least six months: this is so because we considered that NR concomitant with metastasis would not receive any benefit from a nodal irradiation because these patients already have a systemic disease.
The current study was conducted to determine the incidence and risk factors for LRR after adjuvant treatment in patients with early-breast cancer treated with conserving surgery to identify if a subgroup of patients should be treated also with nodal irradiation.
Prognostic factors a like pT, angiolymphatic invasion and G3, more than three positive axillary nodes and age at presentation were independent prognostic factors for LRR Citation[12–15].
Jobsen et al. Citation[16] reported that age less than 40 is the only significant predictor of decreased LRR free-survival (p = 0.001). Moreover the high LRR rate in young women was accompanied by even higher rate of distant metastases Citation[14]. Arriagada et al. observed in patients aged less than 40 years a five-fold increased risk of developing a breast recurrence compared with patients older than 60 years Citation[17], Citation[18]. However, in our series age was an independent prognostic factor for LRR but not for NR. In fact, the only independent prognostic factors for NR were more than three positive lymph nodes, angiolymphatic invasion and pT. However, we found that the percentage of NR in patients with pT2 tumours or presence of angiolymphatic invasion was very low (2.4% and 1.7%, respectively).
The role of regional node irradiation is controversial as different conclusions are noted in different series Citation[19], Citation[20]. In our series for patients with one to three positive nodes, no prognostic factors was found to be significant for NR at the univariate analysis and having one to three positive nodes was not an independent prognostic factor at the multivariate analysis. This result confirmed that for patients with less than three lymph nodes node, irradiation is useless because the incidence of NR is low and it is not influenced by adjuvant treatment. In fact Galper et al. Citation[21] reported that NR is uncommon among patients with negative or ont to three positive lymph nodes treated with conservative surgery, axillary dissection, and only tangential RT fields. Therefore, giving only tangential RT (without a separate nodal field) appears generally acceptable for patients with zero to three positive nodes. In our series the number of negative lymph nodes was not an independent prognostic factor for LRR and NR, in contrast with what had been reported by other authors Citation[22].
Patients with more than three positive nodes are at higher risk of developing LRR and NR. Despite this we found that the percentage of isolated NR in patients with more than three positive nodes was 4.8%. This number is a low percentage of risk to justify a nodal irradiation, also considering the probability of late morbidity after surgery and radiotherapy Citation[23], Citation[24]. Grills et al. Citation[25] referred to 5 and 10-year rates of axillary failure and supraclavicular failure of 0.6% and 1.0% and 0.9% and 1.6%, respectively. Despite this they found a significant benefit for axillary relapses after RT in patients with more than three PAN, the result was not significant for SCR (p = 0.114). Confirming our results for patients with one to three positive axillary nodes: radiotherapy did not affect the rate of axillary failure or supraclavicular failure in patients with one to three positive nodes.
Conclusion
In our series only 1.7% and 4.8% of patients with one to three and more than three PAN, respectively, developed NR as the only site of recurrence. It seams unnecessary to prescribe nodal irradiation to patients with negative or one to three PAN. Regarding patients with more than three PAN, the number of isolated NR is also small to routinely justify node irradiation
References
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-years follow-up a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347(16).
- Fisher B, Anderson S, Briant J, et al. Twenty-years follow-up a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347(16).
- Vinh-Hung V, Burzykowski T, Van de Steene J, et al. Post-surgery in early breast cancer: Survival analysis of registry data. Radiother Oncol 2002; 64: 281–90
- Churn M, Kelly V. Outpatient follow-up after treatment for early breast cancer: Updated results after 5 years. Clin Oncol (R Coll Radiol) 2001; 13: 187–94
- Cowen D, Houvenaeghel G, Bardou V, Jacquemier J, Bautrant E, et al. Local and distant failures after limited surgery with positive margins and radiotherapy for node-negative breast cancer. Int J Radiat Oncol Biol Phys 2000; 47: 305–12
- Voogd AC, Nielsen M, Peterse JL. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: Pooled results of two large European randomized trials. J Clin Oncol 2001; 19(9)2583
- Calle R, Vilcoq JR, Zafrani B, et al. Local control and survival of breast cancer treated by limited surgery followed by irradiation. Int J Radiat Oncol Biol Phys 1986; 12: 873–8
- Delouche G, Bachelot F, Premont M, et al. Conservation treatment of early breast cancer: Long term results and complications. Int J Radiat Oncol Biol Phys 1987; 13: 29–34
- Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–48
- Peto R, Pike MC, Armitage P. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer 1977; 35: 1–39
- Cox DR. Regression models and life-tables. J R Stat Soc B 1972; 334: 187–202
- Fisher BJ, Perera FE, Cooke AL, et al. Extracapsular axillary node extension in patients receiving adjuvant systemic therapy: An indication for radiotherapy?. Int J Radiat Oncol Biol Phys 1997; 38(3)551–9
- Heterlekidis S, Schnitt SJ, Silver B, et al. The significant of extracapsular extention of axillary lymph node metatsases in early-breast cancer. Int J Radiat Oncol Biol Phys 2000; 46(1)31–4
- Pierce LJ, Oberman HA, Strawderman MH, et al. Microscopic extracapsular extension in the axilla: Is this an indication for axillary radiotherapy?. Int J Radiat Oncol Biol Phys 1995; 33(2)253–9
- Mersin H, Yildirim E, Gulben K, et al. Is invasive lobular carcinoma different from invasive ductal carcinoma?. EJSO 2003; 29: 390–5
- Jobsen JJ, van der Palen J, Meerwaldt JH, et al. The impact of age on local control in women with pT1 breast cancer treated with conservative surgery and radiation therapy. Eur J Cancer 2001; 37: 1820–7
- Arriagada R, Le MG, Contesso G, et al. Predictive factors for local recurrence in patients with surgically resected small breast cancer. Ann Oncol ;13 2006; 2002: 1404–13
- Vrieling C, Collette L, Fourquet A, et al. Can patient-treatment-and pathology-related characteristics explain the high local recurrence rate following breast-conserving therapy in young patients?. Eur J Cancer 2003; 39: 932–44
- Recht A, Pierce SM, Abner A, et al. Regional node failure after conservative surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol 1991; 9: 988–996
- Vicini FA, Horwitz EM, Lacerna MD, et al. The role of regional nodal irradiation in the management of patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys 1997; 39: 1069–76
- Galper S, Recht A, Silver B, et al. Factors associated with regional nodal failure in patients with early breast cancer with 0-3 positive axillary nodes following tangential irradiation alone. Int J Radiat Oncol Biol Phys 1999; 45(5)1157–66
- Mersin H, Yildirim E, Buult H. The prognostic significance of total lymph node number in patients with axillary lymph node-negative breast cancer. EJSO 2003; 29: 132–8
- Herd-Smith A, Russo A, Muraca MG, et al. Prognostic factors for lymphedema after primary treatment of breast carcinoma. Cancer 2001; 92(7)1783–7
- Ozaslan C, Kuru B. Lymphedema after treatment of breast cancer. Am J Surg 2004; 187(1)69–72
- Grills IS, Kestin LL, Goldestein N, et al. Risk factors for regional nodal failure after breast-conserving therapy: Regional nodal irradiation reduces rate of axillary failure in patients with four or more positive lymph nodes. Int J Radiat Oncol Biol Phys 2003; 56(3)658–70