Abstract
The clinical value of ultrasonography of the axilla in detection of breast cancer recurrence is not known among patients who have a negative sentinel node biopsy and avoid axillary clearance. We studied a cohort of 205 such patients using ultrasonography one and three years after breast surgery. A recurrent tumour was found in the axilla in only two (0.5%) of the total of 383 ultrasound examinations performed during the study, and only one (0.3%) of the 369 examinations performed at the scheduled study visits revealed cancer. None of the ultrasound examinations was false positive, and no study participant was subjected to unnecessary surgery due to ultrasound monitoring. We conclude that the rate of breast cancer recurrence in the ipsilateral axilla is low following sparing of the axillary contents, and that monitoring of such patients with repeated ultrasound examinations is unlikely to be cost-effective.
The sentinel node biopsy (SNB) has largely replaced axillary lymph node dissection in staging of early breast cancer in many breast surgery units. However, the SNB is associated with a median of a 5% false negative rate Citation[1], and metastases may thus sometimes be left in the axilla when dissection is omitted. Concern has been raised that the proportion of cancer recurrences in the axilla will increase as a result from the use of the SNB, but the frequency of unnecessary surgical explorations of the axilla might also increase due to false positive findings in physical examinations or following false positive ultrasonography (US) of the axilla. Yet, thus far the axillary recurrence rate has been close to zero following a negative SNB during a median follow-up ranging from 22 to 57 months Citation[2–8]. This observation is mainly based on clinical follow-up performed with repeated physical examinations alone Citation[2–8].
Preoperative US adds to the sensitivity of physical examination in detection of axillary lymph node metastases, in particular when combined with examination of fine-needle biopsy aspirates Citation[7], Citation[8]. However, concern has been expressed of increased false positive rates when US monitoring is used as compared to physical examination alone Citation[9]. US used in combination with fine-needle aspiration cytology improves the sensitivity and specificity of detecting lymph node metastases of melanoma during clinical follow-up Citation[10], Citation[11].
Our policy has been to perform physical examination, mammography, and US of the ipsilateral and contralateral axilla one and three years after surgery of the breast in the follow-up of breast cancer patients avoiding axillary clearance following a negative SNB. In the present study we evaluate the risk of regional breast cancer recurrence in such patients. In particular, we assess prospectively the role of routine monitoring of the axilla using US in the clinical follow-up. To the best of our knowledge, little data are currently available regarding the value of US in monitoring of breast cancer patients avoiding axillary clearance following a negative SNB.
Patients and methods
Of the patients diagnosed with invasive breast cancer at the Breast Surgery Units of Helsinki and Espoo, Helsinki University Central Hospital, Finland, between June 2000 and December 2001, 205 had a negative finding in a SNB and were not subjected to axillary clearance. These 205 consecutive patients form the basis of the present prospective study. Patients who had positive sentinel nodes, including those who had only isolated tumor cells in the sentinel nodes, underwent axillary clearance and are thus not included in the present series. The indication for a SNB was considered to be radiologically unifocal, clinically node-negative breast cancer with the largest tumour diameter 3 cm or smaller when measured by breast US. Seven (3%) of the 205 study participants had undergone a preoperative excisional biopsy prior to a SNB and definitive breast surgery. The method of performing SNB and histological examination of the resected tissue are described in a detail elsewhere Citation[12]. The study protocol was approved by the Ethical Committee of Helsinki University Central Hospital. The characteristics of the study participants are presented in .
Table I. Patient and tumour characteristics of the study participants.
Postoperative radiotherapy was given to the residual breast tissue after breast-conserving surgery using a linear accelerator to a cumulative dose of 50 Gy in 25 fractions, and a 10 Gy booster dose was delivered to the operative bed in 5 fractions for premenopausal women. Systemic adjuvant treatment consisted of six 3-weekly cycles of FEC (5-fluorouracil 600 mg/m2, epirubicin 60 mg/m2, and cyclophosphamide 600 mg/m2, or of three 3-weekly cycles of docetaxel (80 to 100 mg/m2), or either eight weekly cycles of vinorelbine (25 mg/m2) or 3 weekly cycles of docetaxel (80 to 100 mg/m2) followed by three 3-weekly cycles of FEC given within a context of a prospective randomized trial. Women with estrogen receptor (ER) or progesterone receptor (PgR) positive cancer received tamoxifen 20 mg daily for 5 years. Planned follow-up visits took place at one and three years after breast surgery. Physical examination, blood cell counts and blood chemistry, bilateral mammography, and US of the axilla were performed at these visits. The study participants had access for extra visits at the Department of Oncology whenever there was concern of breast cancer recurrence.
The US examination of the breast and the ipsilateral axilla was carried out or supervised by a senior radiologist. Either an Echo Camera SSD-680 Aloka ultrasound system (Aloka Company Ltd., Tokyo, Japan) with a 7.5 MHz linear array transducer or a Toshiba Power Vision 6000, SSA-370A (Toshiba Company Ltd., Tokyo, Japan) was used.
The ultrasonographic features considered as suspicious for malignancy in an axillary lymph node were two-dimensional enlargement giving a rounded appearance of the lymph node, an echo-poor central hilus, and eccentricity of the nodal cortex. If at least one of these features was present, a US-guided fine-needle aspiration biopsy was performed. Clinically suspicious nodes and those suspicious in a US examination, and nodes with atypical, suspicious, or malignant cytological findings were excised surgically for histological examination. All axillary recurrences were histologically confirmed.
Results
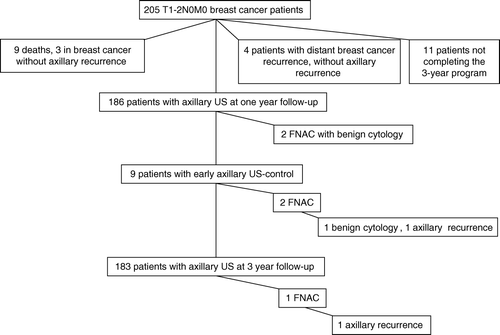
Nine patients died during the follow-up period, of whom three from breast cancer without signs of axillary recurrence. Four patients developed distant breast cancer recurrence without findings of axillary recurrence. Eleven other patients did not complete the 3-year follow-up program, but all were alive at the end of the study according to the files of the Finnish Cancer Registry.
At the 12-month follow-up visit 186 (93%) of the 201 study participants had US examination of the axilla. No breast cancer recurrences were detected in these or other examinations. Two patients had a fine-needle aspiration cytology taken due to presence of enlarged, but ultrasonographically nonsuspicious axillary lymph nodes. Fine-needle aspirates contained only benign cells, and both patients had subsequently two follow-up US examinations with normal findings.
At the 3-year follow-up visit 183 (98%) of the 186 study participants had axillary US examination performed. One patient had suspicious axillary lymph nodes in US, and presence of breast cancer was confirmed both cytologically from a fine-needle biopsy aspirate and histologically from the tissue removed at surgery. No other needle or excisional biopsies were taken from any of the patients, but one patient had one repeat US examination following detection of enlarged, ultrasonographically nonsuspicious lymph nodes.
Nine patients had axillary US performed between the scheduled study visits, six patients due to axillary pain, two due to presence of palpable nodes, and one patient visited the study centre one year too early by a mistake. Fine-needle aspiration cytology was taken from two of these patients, and revealed breast cancer in the patient who visited the study centre one year too early. Thus, breast cancer was detected in only two (0.5%) of the total of 383 US examinations performed during the study, and in only one (0.3%) of the 369 examinations performed at the scheduled visit times. The outcome of the ultrasonographic follow-up program is summarized in .
Discussion
The present findings suggest that the breast cancer recurrence rate is low in the ipsilateral axilla when axillary dissection is omitted following a negative SNB. In several studies the sensitivity of the US has been found to be greater than that of clinical examination in the detection of lymph node metastases Citation[8], Citation[13], Citation[14]. In the present series nonpalpable cancer recurrence in the axillary lymph nodes was discovered by US in two patients, but less than 1% of the US examinations resulted in detection of breast recurred cancer.
A few studies suggest that the use of axillary US results in no overall diagnostic improvement due to an increased rate of false positive findings Citation[9], Citation[15]. We found enlarged, although morphologically not suspicious nodes only in three of the scheduled US studies. Even though fine-needle aspiration cytology was normal in two of these cases, the specificity of the US examination was still relatively high in the present study Citation[10], Citation[11]. We had no false positive findings in fine-needle aspiration cytology, which is in line with studies that suggest a generally high specificity for fine-needle aspiration in the diagnosis of breast cancer Citation[16].
Two-dimensional enlargement giving a rounded appearance to the lymph node, an echo poor central hilus, and eccentricity of the nodal cortex have traditionally been considered as features suspicious for metastasis in US Citation[8], Citation[17], Citation[18]. The size of the lymph nodes may be of less importance than anticipated Citation[19]. In a recent study on preoperative US examination of the axilla the maximum lymph node cortex thickness was the most important feature that predicted metastatic involvement Citation[20].
The diagnostic accuracy of US to detect axillary metastases of breast cancer depends on many factors, such as experience and skill of the surgical team to perform the sentinel node biopsy, the skill and equipment of the radiologists who performs axillary US examinations, the frequency and technique of locoregional radiation therapy given, the use of systemic cancer therapy, the frequency of follow-up visits, and the patient population studied. The clinical value of longitudinal US examinations in follow-up of breast cancer patients and its effect on survival could most reliably be evaluated in a randomized controlled study where a part of the study participants were monitored with US and others were not. However, the findings of the present study suggest that such a randomized study may not be easy to perform. The number of nonpalpable recurrent tumours detected by US is likely to be low in experienced centres, some of the cancers with axillary metastases detected early by US may already have given rise to distant metastases, and some cancers may not threaten life even when axillary metastases have grown palpable, suggesting that a very large clinical study would be required to show a significant difference in survival, if there is any.
We conclude that the risk of axillary recurrence after omitting axillary dissection is low in patients whose axillary clearance has been omitted following a negative SNB during the first three years following breast surgery. Serial monitoring of the ipsilateral axilla using US resulted only in a few needle biopsies, and rarely in repeat US examinations. We detected no false positive ultrasonographic or fine-needle biopsy findings, and none of the patients was subjected to unnecessary surgery of the axilla due to US monitoring. However, the detection rate of axillary cancer recurrence was very low, less than 1%, which suggests that routine monitoring of the ipsilateral axilla using US is not worthwhile among breast cancer patients whose axilla has been left undissected after a negative SNB.
References
- Miltenburg DM, Miller C, Karamlou TB, Brunicardi FC. Meta-analysis of sentinel lymph node biopsy in breast cancer. J Surg Res 1999; 84: 138–42
- Chung MA, Steinhoff MM, Cady B. Clinical axillary recurrence in breast cancer patients after a negative sentinel node biopsy. Am J Surg 2002; 184: 310–4
- Schrenk P, Hatzl-Griesenhofer M, Shamiyeh A, Waynad W. Follow-up of sentinel node negative breast cancer patients without axillary lymph node dissection. J Surg Oncol 2001; 77: 165–70
- Roumen RM, Kuijt GP, Liem IH, van Beek MW. Treatment of 100 patients with sentinel node-negative breast cancer without further axillary dissection. Br J Surg 2001; 88: 1639–43
- Giuliano AE, Haigh PI, Brennan MB, Hansen NM, Kelley MC, Ye W, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol 2000; 18: 2553–9
- Veronesi U, Galimberti V, Mariani L, Gatti G, Paganelli G, Viale G, et al. Sentinel node biopsy in breast cancer: Early results in 953 patients with negative sentinel node biopsy and no axillary dissection. Eur J Cancer 2005; 41: 231–7
- Torrenga, H, Fabry, H, van der, Sijp JR, van Diest, PJ, Pijpers, R, Meijer, S. Omitting axillary lymph node dissection in sentinel node negative breast cancer patients is safe: A long term follow-up analysis. J Surg Oncol 2004;88:4–7; discussion 7–8.
- Naik, AM, Fey, J, Gemignani, M, Heerdt, A, Montgomery, L, Petrek, J, et al. The risk of axillary relapse after sentinel lymph node biopsy for breast cancer is comparable with that of axillary lymph node dissection: A follow-up study of 4008 procedures. Ann Surg 2004;240:462–8; discussion 468–71.
- Verbanck J, Vandewiele I, De Winter H, Tytgat J, Van Aelst F, Tanghe W. Value of axillary ultrasonography and sonographically guided puncture of axillary nodes: A prospective study in 144 consecutive patients. J Clin Ultrasound 1997; 25: 53–6
- Pamilo M, Soiva M, Lavast EM. Real-time ultrasound, axillary mammography, and clinical examination in the detection of axillary lymph node metastases in breast cancer patients. J Ultrasound Med 1989; 8: 115–20
- Tate JJ, Lewis V, Archer T, Guyer PG, Royle GT, Taylor I. Ultrasound detection of axillary lymph node metastases in breast cancer. Eur J Surg Oncol 1989; 15: 139–41
- Leppänen E, Leidenius M, Krogerus L, von Smitten K. The effect of patient and tumour characteristics on visualization of sentinel nodes after a single intratumoural injection of Tc 99m labelled human albumin colloid in breast cancer. Eur J Surg Oncol 2002; 28: 821–6
- Blum A, Schlagenhauff B, Stroebel W, Breuninger H, Rassner G, Garbe C. Ultrasound examination of regional lymph nodes significantly improves early detection of locoregional metastases during the follow-up of patients with cutaneous melanoma: Results of a prospective study of 1288 patients. Cancer 2000; 88: 2534–9
- Rossi CR, Seno A, Vecchiato A, Foletto M, Tregnaghi A, De Candia A, et al. The impact of ultrasound scanning in the staging and follow-up of patients with clinical stage I cutaneous melanoma. Eur J Cancer 1997; 33: 200–3
- Bruneton JN, Caramella E, Hery M, Aubanel D, Manzino JJ, Picard JL. Axillary lymph node metastases in breast cancer: Preoperative detection with US. Radiology 1986; 158: 325–6
- Kuenen-Boumeester V, Menke-Pluymers M, de Kanter AY, Obdeijn IM, Urich D, Van Der Kwast TH. Ultrasound-guided fine needle aspiration cytology of axillary lymph nodes in breast cancer patients. A preoperative staging procedure. Eur J Cancer 2003; 39: 170–4
- Sapino A, Cassoni P, Zanon E, Fraire F, Croce S, Coluccia C, et al. Ultrasonographically-guided fine-needle aspiration of axillary lymph nodes: Role in breast cancer management. Br J Cancer 2003; 88: 702–6
- Lernevall A. Imaging of axillary lymph nodes. Acta Oncol 2000; 39: 277–81
- Obwegeser R, Lorenz K, Hohlagschwandtner M, Czerwenka K, Schneider B, Kubista E. Axillary lymph nodes in breast cancer: Is size related to metastatic involvement?. World J Surg 2000; 24: 546–50
- Deurloo EE, Tanis PJ, Gilhuijs KG, Muller SH, Kroger R, Peterse JL, et al. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur J Cancer 2003; 39: 1068–73