Abstract
We studied the effect of postoperative radiotherapy (RT) after breast sector resection for ductal carcinoma in situ (DCIS). The study protocol stipulated radical surgery but microscopically clear margins were not mandatory. We randomised 1 046 operated women to postoperative RT or control between 1987 and 1999. The primary endpoint was ipsilateral local recurrence. Secondary endpoints were contralateral breast cancer, distant metastasis and death. After a median follow-up of 5.2 years (range 0.1–13.8) there were 44 recurrences in the RT group corresponding to a cumulative incidence of 0.07 (95% confidence interval (CI) 0.05–0.10). In the control group there were 117 recurrences giving a cumulative incidence of 0.22 (95% CI 0.18–0.26) giving an overall hazard ratio of 0.33 (95% CI 0.24–0.47, p < 0.0001). Twenty two percent of the patients had microscopically unknown or involved margins. We found no evidence for different effects of RT on the relative risk of invasive or in situ recurrence. Secondary endpoints did not differ. Women undergoing sector resection for DCIS under conditions of population based screening mammography benefit from postoperative RT to the breast. Seven patients needed RT-treatment to prevent one recurrence.
The National Board of Health and Welfare in Sweden issued recommendations for mammography screening in early 1986. In Sweden the question was raised whether postoperative radiotherapy (RT) after breast conserving surgery for ductal carcinoma in situ (DCIS) would have a clinically relevant effect in a biological domain where the majority of lesions would be mammographically detected and excised with the standardized sector resection Citation[1] that had been the recommended surgical technique in Sweden since the 1980's.
In 1987, the Swedish Breast Cancer Group launched a randomised trial comparing postoperative RT versus no such treatment in women who had undergone breast conserving surgery for DCIS. In 1999 the trial was closed after inclusion of 1 067 women. We report the findings after a mean follow-up of 5.4 years (median follow-up was 5.2 years). The primary endpoint was ipsilateral in situ or invasive breast recurrence. Secondary endpoints were ipsilateral regional recurrence, contralateral breast cancer, distant metastasis, death of all causes and death from breast cancer. The presentation follows the CONSORT recommendations Citation[2].
Patients and methods
Enrolment criteria
To be eligible, women had to be operated with breast conserving surgery for histologically proven DCIS occupying a quadrant or less of the breast. A clinically negative examination of the axilla was mandatory for inclusion. Patients who for reasons of age, mental, social or medical status were expected not to cope with the interventions were not eligible. Exclusion criteria were Paget's disease of the nipple, invasive carcinoma or intracystic carcinoma in situ, ongoing pregnancy or a history of previous or concurrent malignancy (except basal cell carcinoma and treated carcinoma in situ of the uterine cervix).
Ethics
Ethical permission was granted for the study in all Swedish health care regions. Full informed consent was required.
Randomisation
Women were randomly assigned to RT or control. Allocation to the different arms was given to the treating physician via telephone from one of six regional Oncologic Centres. The randomisation was stratified for health care region and permutated in blocks of four, a number unknown to the trialists. Base-line data were reported by the treating physician.
Interventions
Patients underwent a sector resection Citation[1] from Scarpa's fascia ventrally and dorsally including the pectoral fascia encompassing the entire lesion and macroscopic lateral and medial margins of at least one centimetre were aimed for. Specimen x-ray was compulsory. Histopathology was based on routine reports. Microscopically radical removal of the lesion was not required. The study protocol stated that “due to the multicentric nature of DCIS “microscopic radicality” cannot always be achieved”. Radical removal of DCIS was finally judged by the operating surgeon based on operative findings, specimen radiography and pathology reports. The surgeon then opted for further surgery or study inclusion. RT was given in the supine position. The target volume was the remaining breast parenchyma as defined by palpation, the position of the surgical scars and available radiographs. The scars and the part or the chest wall underlying the excised specimen were included in the target volume. No boost radiation was given to the tumour bed. The specification of the absorbed dose was according to the ICRU Report 50 Citation[3]. The treatment could be given either continuously or as a split course treatment. The specification dose was 50 Gy given in 25 fractions over five weeks or 54 Gy given in two series with a gap of two weeks. The dose in the specification point should be 15.5 according to the CRU-formulation which should be used to correct for departures of doses or overall time. Of the 485 patients who received RT, split course RT was given in 35 (7%) and 61 patients (12.6%) were treated using 2.4 Gy four times a week to 48 Gy (CRE 15.6). The remaining 389 patients were treated with 50 Gy given in 25 fractions over five weeks. A total of 33 patients, balanced between the groups (), received anti-estrogen therapy for two years.
Table I. Patient characteristics by study arm.
Follow-up
Patients were initially followed by biannual clinical examination and yearly mammography for five years and thereafter by clinical examination and mammography on a yearly basis. After closure of the study (December 31, 1999), the last follow-up date was set to July 31, 2001. All patients’ records were then scrutinized regarding mammography reports, operative findings, specimen x-rays and radiotherapy reports, data on adjuvant treatments and original pathology reports. The clinical database was locked in March 2003 and the histopathological re-evaluation was completed in October 2003.
Clinical events
All events in the ipsi- or contralateral breast or in the ipsilateral axilla were based on clinical findings or mammography and verified by histology and classified as either in situ or invasive carcinoma or both. Distant metastases were diagnosed by scintigram, radiographs or computed tomographic scans. When findings with imaging techniques were doubtful microscopic confirmation was performed if possible. Lymph node metastases beyond the axilla were mostly confirmed by fine-needle aspiration cytology. Causes of death were extracted from the Swedish Cause of Death Registry. Death from other causes but with residual cancer was classified as cancer deaths. Incidence and death from other malignancies were not sought for and are hence not included in the analysis.
Histopathology re-evaluation
To investigate the validity of the initial diagnosis 212 (20%) patients were selected by day of birth (sets of six different days in each region) after study closure. It was agreed that the definition of DCIS should follow the Consensus Conference on the classification of DCIS Citation[4] and its nuclear grade and accordingly DCIS grade I and II <2 mm were classified as atypical ductal hyperplasia (ADH) Citation[5], Citation[6]. From this cohort random sample the histological slides were retrieved.
Statistics
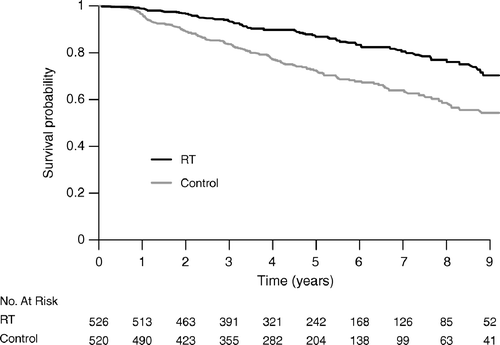
A number of 500 patients per study arm were needed to detect an absolute difference of 10% in local recurrence risk rate at five years at a 5% significance level with a power of at least 90%, using a log-rank test Citation[7]. The primary endpoint of the study was time to ipsilateral breast recurrence, ignoring contralateral cancer and censoring for distant recurrence and death. Secondary endpoints were: 1) Time to contralateral cancer ignoring ipsilateral breast recurrence and censoring for distant recurrence and death; 2) Disease-free survival, i.e. time to the first of the events ipsilateral breast recurrence, contralateral cancer, distant recurrence or death; 3) Time to the first of the events distant recurrence or breast cancer death ignoring ipsilateral breast recurrence and contralateral cancer and censoring for other deaths. Log-rank tests and Cox regression analyses stratified for health care region were used to compare event rates between the treatment groups. Cumulative incidence curves Citation[8] were used to illustrate ipsilateral recurrences as first events in the presence of other recurrences and death and to determine the cumulative incidence of contralateral cancer with distant recurrences and death as competing events. We calculated the absolute differences in cumulative incidence. Kaplan–Meier curves were used to illustrate event-free survival. All analyses were done according to intention-to-treat, and all confidence intervals and significance tests were two-sided.
Results
Participants
Between September 1987 and December 31, 1999, 1 067 patients were randomised either to RT (n = 534) or control (n = 533). Fifty-eight departments enrolled from one to 70 patients into the study. In the monitoring process 20 patients were excluded, seven in the RT group and 13 in the control group. Exclusions were done only when the information available at the time of randomisation showed that the protocol was violated (see for details). Forty-two of the 527 allocated to RT did not receive RT and five patients allocated to control received RT for different reasons (). No patient was lost to follow-up and 1 046 patients were analyzed.
Figure 1. Patient flow according to CONSORT recommendations Citation[2]. After exclusions for protocol violations randomised patients were analyzed according to intention to treat.
![Figure 1. Patient flow according to CONSORT recommendations Citation[2]. After exclusions for protocol violations randomised patients were analyzed according to intention to treat.](/cms/asset/702d63d3-ff55-4b28-845d-ca3182324473/ionc_a_168126_f0001_b.gif)
Baseline characteristics
Baseline characteristics were well balanced between the groups (). Specimen x-ray was used during 96.9% of the operations.
Histopathological re-evaluation
Slides from fourteen of the 212 cases (7%) could not be found. The remaining 198 cases were evaluated by three pathologists. In 62 cases there were discrepancies concerning the diagnosis between the pathologists but consensus was reached in all but nine cases where the majority ruled. Of the 198 cases, 82% (163/198) were classified as DCIS. The 35 cases that were not DCIS were classified as benign 6.6% (13/198), atypical ductal hyperplasia (ADH) in 5% (10/198), invasive/microinvasive cancers in 4% (5 + 3/198), lobular carcinoma in situ in 0.5% (1/198) and in 1.5% (3/198) the material was insufficient for a conclusive diagnosis. Margins could not be re-assessed at the time of re-evaluation. The number of patients in whom the margins were given in the original pathology report is shown in .
Events
There were 161 ipsilateral recurrences, 44 in the RT group and 117 in the control group (). The hazard ratio for ipsilateral recurrence for patients given RT compared to the control group was 0.33 (95% CI 0.24–0.47). When the ipsilateral recurrences were further analyzed () there were 92 recurrent DCIS, 23 in the RT-group and 69 in the control group (Hazard ratio 0.31, 95% CI 0.20–0.50). There were altogether 69 ipsilateral invasive breast recurrences, 21 in the group treated with RT and 48 in the control group and (Hazard ratio 0.41, 95% CI 0.24–0.69). The cumulative incidence of ipsilateral breast recurrences is shown in . The five year cumulative incidence of local recurrence was 7% (95% CI 5%–10%) in the RT group and 22% (95% CI 18%–26%) in the control group and the overall hazard ratio was 0.33 (95% CI 0.24–0.47) (). Similarly the hazard ratio for patients with clear specimen pathology margins was 0.35 (95% CI 0.23–0.52) and the combined hazard ratio for women with either specimen pathology margins unknown or positive was 0.31 (95% CI 0.16–0.60). However, in the subgroup with unclear margins the baseline risk was high for those not irradiated and thus the absolute risk reduction following RT substantial −37% at eight years.
Figure 2. Cumulative incidence of all ipsilateral recurrences. The number of patients at risk is listed at the bottom of the figure. p < 0.0001, log-rank test.
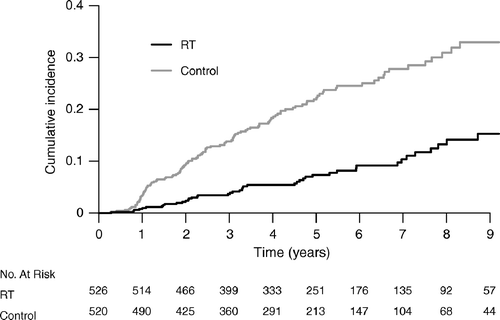
Table II. Number of events according to study definitions of endpoints, cumulative incidence, difference in cumulative incidences and hazard ratios with their respective 95% confidence intervals.
Table III. Distribution within each study endpoint of events by type (in situ or invasive events, occurrences of distant metastases and breast cancer deaths).
The Hazard ratio for allocation to RT versus control was 0.40 (95% CI 0.16–0.99, p = 0.049) when the analysis was restricted to those 163 women who had a verified DCIS in the 20% sample for histopathological re-evaluation.
Population-based mammography screening was implemented stepwise in Sweden. We therefore analyzed the data regarding all endpoints during three different time periods; one period where prevalent screening prevailed, one period when screening was a mixture of prevalent and incidence screening and finally a period consisting of incidence screening. No difference in relative effect of RT was found between the three time periods (data not shown).
Ipsilateral regional recurrences in the axilla were scarce and only one recurrence in the control group appeared as a first event (patient included in the invasive recurrence group, ). There were altogether 48 contralateral events ( and ), 26 in the RT group and 22 in the control group. Contralateral DCIS amounted to ten events, three in the RT group and seven in the control group () and invasive contralateral breast cancers amounted to 38, 23 in the RT group and 15 in the control group.
Eighteen women developed distant metastasis or died from breast cancer, and the events were almost equally distributed between the groups ( and ). Sixteen of the 18 patients had an invasive recurrence diagnosed preceding distant metastases. In the overall analysis of event-free survival () there was a statistically significant difference between the study groups with far less events in the RT group (p < 0.001 log-rank test).
Discussion
RT gave a substantial reduction of the risk of ipsilateral recurrence during a five year follow-up. This reduction was seen in a clinical domain where most women were recruited from a population exposed to service screening mammography and who all had undergone a standardized sector resection. We found no evidence for different effects of RT on the risk of invasive or in situ recurrence. There was neither an effect of RT on the incidence of contralateral breast events nor was there a discernible effect of RT on distant metastases or overall survival, the follow-up time, however, is too short to expect any differences on the latter matters.
The randomisation resulted in balanced groups. We achieved a complete follow-up and the study was completely monitored via original patient records. Follow-up both in terms of clinical visits and mammography was similar in both groups. There was a high statistical power in relation to the primary endpoints of the study. Re-evaluation of histopathological slides of a random sample of 20% of the whole study group showed that 12% of the patients had benign lesions or atypical ductal hyperplasia and another 4% had invasive/microinvasive cancer. This is to be expected as the study used routine histopathology for inclusion. The NSABP-B17 histopathology report Citation[9] showed that 73% were DCIS, 7% were benign lesions, 2% were invasive cancers and 21% the material was judged inadequate for analysis. The EORTC 10853 study retrieved 85% of their slides for review and 90% of the retrieved patients were reported as having DCIS Citation[10].
It is hard to make predictions how our misclassification, which was both at the benign and the invasive ends of the DCIS-spectrum, may have affected our estimates of the absolute risk levels and the misclassifications should not bias the comparison between the arms. The other three published randomised studies on DCIS±RT Citation[11–13] have all used routine pathology as a basis for their original reports. Two of the three studies have in subsequent reports re-evaluated the histopathology Citation[9], Citation[10] and both stated that the number of recurrences per treatment arm was similar for reviewed and non-reviewed cases. A separate analysis of our re-evaluated 163 DCIS cases yielded similar results as compared to our overall estimates.
From detailed studies in one of our regions Citation[14] we infer that around half of the eligible patients were entered into the trial, which is a high proportion of included patients. We judge that the overall mammography quality was high since several centres in Sweden had participated in randomized mammography trials and Swedish mammograhers thus had good access to experienced training centres. In 1986 the National Board of Health and Welfare issued guidelines for mammography screening, including demands on quality control both in terms of interpretation of films and technical quality.
The study protocol demanded macroscopically free surgical margins in relation to any clinically or mammographically detectable lesion, but histopathologically clear margins were not a prerequisite. In our monitoring process we found that the documentation of margins either by histology or specimen x-ray was insufficient in 17–21% of the medical records. However, this does not mean that margins were close or involved in a similar proportion. Specimen radiography was done in 97% in the operations with intraoperative answers to the surgeon. Since clear microscopic margins has been demanded for breast conserving surgery for invasive cancers throughout the period and since involved margins has been discussed as a risk factor during the course of the study, most surgeons probably opted for histopathologically clear margins as indicated by re-excisions in 8% of the patients – a figure similar to that of the proportion of patients with documented involved margins. If insufficient documentation of margins still often indicate involved margins and would be a strong determinant of risk of local failure our results may show higher absolute risks than if histopathologically totally free margins had been required. It is not clear however, how the proportion of patients with unclear margins would influence the relative effects of RT. Our analysis of the subgroups with histopathologically radically removed lesions and lesions with unknown showed similar relative effect of RT. Patients with involved margins generally have a higher relapse risk and there is no evidence that this can be compensated for by RT. Hence we think that a complete surgical removal, with a safety margin, of all ducts involved with carcinoma in situ is an important part of handling DCIS.
However, Bijker et al. have reviewed the results of the EORTC 10853 study concerning local recurrence and found when comparing specimens that reported microscopically free margins and specimens with close/involved margins and margins not reported a hazard ratio of 2.07 (95% CI 1.35–3.16) for recurrence Citation[10]. Studies in invasive cancer indicate that the relative effects of RT are similar in trials with quite different types of surgery performed, but the radicality of the surgery may influence the absolute risk levels Citation[15].
The inclusion period for the study was long. It is possible that length bias sampling might have been more pronounced in the beginning of the study period when prevalence screening dominated and that mammography quality changed with increased experience and technical development. The lack of any time trends in our study indicates that the results probably can be generalized to patients diagnosed today.
Our study results concerning the relative effects of RT on all ipsilateral events are similar to those previously reported Citation[11–13], Citation[16]. Looking specifically at the effects on in situ versus invasive recurrences, our study coincides with the UK/ANZ and the EORTC 10853 study that showed that RT is equally protective for both events. The NSABP B-17 repeatedly showed a somewhat different protective effect of RT inasmuch as DCIS-recurrences were twice as many as invasive recurrences Citation[11], Citation[16]. We have no obvious explanation for this difference.
Direct comparisons of the incidence of ipsilateral recurrences between the above mentioned published randomised studies are difficult to make since the definition of DCIS including inclusion of patients with “microinvasive DCIS”, the definition of radical removal and statistical methods differs between the randomized studies. Moreover the follow-up times differ and the populations studied in the different studies and accrual rates in relation to the total number of eligible patients are far from uniform between studies.
When analysing time to the primary endpoint, ipsilateral recurrence, we ignored contralateral breast cancers but censored follow-up if the women got metastases. The reason for this choice is that contralateral breast cancers most often are new primaries with a natural history independent of the first primary and that search for ipsilateral recurrences also would continue after a contralateral cancer. Metastases on other hand, are events along the same course of natural history as the primary tumour and ipsilateral recurrences, and thus not independent of these. Furthermore, search for ipsilateral recurrences is not prioritized after diagnosis of distant metastasis and the risk of local recurrence may also be modified of treatments for advanced disease. The cumulative incidence function of ipsilateral recurrences considers the competing events distant metastases and death correctly Citation[8].
Postoperative RT gives a substantial relative reduction and a clinically meaningful absolute risk reduction of local recurrence at a similar level also in a clinical domain with women mainly recruited from a population offered mammography screening. Our results show a number of seven patients needed to treat to prevent one local recurrence after five years of follow-up.
We thank the Swedish Cancer Society for financial support and North Sweden Clinical Research Institute that, on the authors’ mission, externally reviewed all Clinical Report Forms according to Good Clinical Practice. K. Aspegren, A. Taube, I. Idvall, S. Thorstenson. G. Juhlin, K. Strömgren and I. Backlund all contributed to various aspects of the work.
References
- Aspegren K, Holmberg L, Adami HO. Standardization of the surgical technique in breast-conserving treatment of mammary cancer. Br J Surg 1988; 75: 807–10
- Moher D, Schulz KF, Altman DG. The CONSORT Statement: Revised Recommendations for Improving the Quality of Reports of Parallel-Group Randomized Trials. Lancet 2001; 357: 1191–4
- ICRU: Prescribing and Reporting Photon Beam Therapy. Report # 50. Washington: International Commission on Radiation Units and Measurements; 1993.
- Consensus conference on the classification of ductal carcinoma in situ. Human Pathology 1997;28:1221–5.
- Tavassoli FA, Norris HJ. A comparison of the results of long term follow-up for atypical intraductal hyperplasia and intraductal hyperplasia of the breast. Cancer 1990; 65: 518–29
- Elston CW, Ellis IO. The Breast. Systemic Pathology 13. Churchill Livingstone, Edinburgh 1998
- Machin D, Campbell MJ. Statistical tables for the design of clinical trials2nd ed. Blackwell Science, Oxford 1997
- Kalbfleisch DL, Prentice RL. The statistical analysis of failure time data2nd ed. John Wiley, New York 2002
- Fisher ER, Costantino J, Fisher B, Palekar AS, Redmond C, Mamounas E. Pathologic findings from the National Surgical Adjuvant Breast Project Protocol B-17. Cancer 1995; 75: 1310–9
- Bijker N, Peterse JL, Duchateau L, Julien JP, Fentiman IS, Duval C, et al. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ: Analysis of European Organization for Research and Treatment of Cancer Trial 10853. J Clin Oncol 2001; 19: 2263–71
- Fisher B, Costantino J, Redmond C, Fisher E, Margolese R, Dimitrov N, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med 1993; 328: 1581–6
- Julien JP, Bijker N, Fentiman IS, Peterse JL, Delledonne V, Rouanet P, et al. Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: First results of the EORTC randomised phase III trial 10853. EORTC Breast Cancer Cooperative Group and EORTC RT Group. Lancet 2000; 355: 528–33
- Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: Randomised controlled trial. Lancet 2003; 362: 95–102
- Ringberg A, Moller T. Accrual rate-limiting factors in a Swedish randomised ductal carcinoma in situ (DCIS) trial – a demographic study. Eur J Cancer 2000; 36: 483–8
- Veronesi U, Volterrani F, Luini A, Saccozzi R, Del Vecchio M, Zucali R, et al. Quadrantectomy versus lumpectomy for small size breast cancer. Eur J Cancer 1990; 26: 671–3
- Fisher B, Dignam J, Wolmark N, Mamounas E, Costantino J, Poller W, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol 1998; 16: 441–52
Appendix
Conflict of interest statement
None declared. All authors took part in interpretation of the data and writing the manuscript. In addition the following special responsibilities were executed:
Protocol committee. L. Holmberg, A. Wallgren (chairman).
Steering committee. H. Anderson, L. G. Arnesson, S. O. Emdin, B. Granstrand, L. Holmberg (chairman), H. Nordgren, A. Ringberg, K. Sandelin, A. Wallgren.
Statisticians. H. Anderson (chairman), H. Garmo.
Pathology evaluation. H. Nordgren (chairman), A. Ringberg (coordinator).
Data management and monitoring. S. O. Emdin, B. Granstrand (coordinators), L. Holmberg (chairman), A. Ringberg.
Statistical validity. Two of the authors (HA and HG) are graduated bio-statisticians working fulltime in the settings of epidemiology and clinical trials.