Abstract
The treatment strategy for malignant liver tumors should be appropriately determined because post-treatment quality of life greatly depends on the patients’ residual hepatic function. In this report, we present three patients with malignant liver tumors treated by proton beam therapy in whom pre- and post-therapeutic hepatic functional reserves were evaluated sequentially for more than a year by 99mTechnetium-galactosyl human serum albumin (99mTc-GSA) scintigraphy. All three patients exhibited the distinctive time course of 99mTc-GSA uptake efficiency, which suggested a transient decline in the ratio of liver activity to heart and liver activity at 15 minutes (LHL15) 3–6 months after proton beam therapy. This change was not in parallel with that expected from a functioning normal liver tissue volume. In a year after proton beam therapy, LHL15 recovered nearly to the pre-treatment level in all three patients. Our observations may be related to the up-regulation of receptor-mediated 99mTc-GSA uptake during hepatic regeneration after proton beam therapy.
There are various treatment modalities for malignant liver tumors, such as partial hepatectomy, percutaneous ethanol injection therapy (PEIT), radiofrequency ablation (RFA), transcatheter arterial embolization (TAE), and transcatheter arterial or systemic chemotherapy. As for primary hepatocellular carcinoma (HCC), most of the patients have poor hepatic functions due to chronic hepatitis C or B virus infections with liver cirrhosis Citation[1], Citation[2]. Metastatic liver tumors are often found with multiple lesions at the time of diagnosis Citation[3], Citation[4]. Accordingly, it is important to preserve the uninvolved normal liver after treatment whether the tumor is primary or metastatic.
The treatment strategies should be determined not only by the patients’ general condition and tumor status, but also by the post-therapeutic hepatic functional reserve. We have treated patients with liver tumors using proton beam therapy since 1985. A proton beam is a charged-particle radiation characterized by a well-defined range of penetration with a deep-seated peak of energy deposit which is called the Bragg peak Citation[5], Citation[6]; higher doses can be delivered to the tumors, whereas doses to adjacent normal structures are restricted to tolerable levels, compared with conventional photon radiotherapy Citation[7], Citation[8]. The treatment modality of our institution is potentially beneficial, because it is non-invasive and can achieve a high local control rate for liver tumors as high as 86.9% at 5 years Citation[9]. Our previous studies have revealed its efficacy and feasibility as a single treatment modality for HCC not only under conditions in which other treatment modalities are also available, but also under unfavourable conditions such as large tumors or those with portal vein tumor thrombus Citation[9–11]. Recently, other proton beam therapy institutions reported treatment results for HCC similar to ours Citation[12], Citation[13].
In the present study, we report the sequential changes in the hepatic functional reserve as evaluated by 99mTc-GSA scintigraphy before and after proton beam therapy in three patients with liver tumors Citation[14]. The distinctive courses of the hepatic functional reserve were consistently noted among all three patients, a finding that is not consistent with what is expected in a normal liver tissue volume change assessed by x-ray computed tomography (CT) images. Written informed consent was obtained from these three patients for proton beam therapy and follow-up evaluations.
Case 1
A 67-year-old male without evidence of hepatitis virus infection with a history of heavy alcoholic consumption consulted us at our hospital for proton beam therapy as an additional treatment after TAE for a solitary 13-cm HCC lesion in the right lobe of the liver in May 2003. At his first visit, the serum AFP level was 32 597 ng/mL without liver cirrhosis (A). He received 66 Gy of proton beam therapy in 22 fractions over 38 days (B).
Figure 1. CT and 99mTc-GSA scintigraphy SPECT images of Case 1. (A) Pre-treatment enhanced CT image with a large nodule occupying almost the entire right lobe of the liver. (B) Dose distribution of the proton beam therapy. The clinical target volume was contoured on the CT images. (C) The enhanced CT image in the arterial phase at six months after proton beam therapy. The enhancement in the arterial phase was diminished, and the surrounding normal liver tissue was enhanced as a sign of radiation hepatitis. Atrophic change in the irradiated normal liver tissue had already started. Ascites appeared around the tumor with no other clinical symptoms of hepatic failure. Before (D), at the end of (E), and 6 (F), and 12 (G) months after proton beam therapy. An uptake defect in liver S6/7, which corresponded to the tumor, was observed before proton beam therapy (D). At the end of the therapy, the extent of this uptake defect was greater because the radiation damage decreased 99mTc-GSA uptake in the irradiated normal liver tissue around the tumor (E). After the therapy, the uptake defect continued to exist, and 99mTc-GSA uptake was high in the left lobe of the liver (E–G). At six months, the compensatory hypertrophy process appeared to be prominent, because 99mTc-GSA uptake of the entire left lobe was homogeneously high (F). At twelve months after proton beam therapy, 99mTc-GSA uptake in the dorsal area of the left lobe had relatively increased (G) and resembled the pre-treatment uptake distribution of the left lobe (D). This finding suggested that the compensatory hypertrophy process had calmed down over the course of one year.
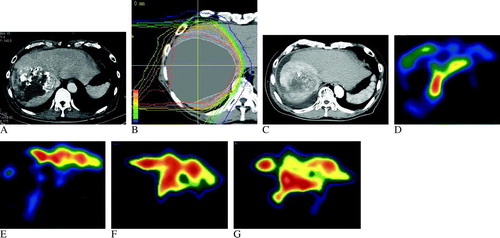
Six months after the proton beam therapy, the tumor stain became vague, which suggested decreased tumor vascularity and viability after proton beam therapy, with a sign of radiation hepatitis in the surrounding irradiated normal liver tissue, and compensatory hypertrophy of the left lobe of the liver was observed in CT images (C) Citation[15]. CT findings of radiation hepatitis were also seen one year after the proton beam therapy. The irradiated normal liver tissue became atrophic six months after the proton beam therapy, and the tumor and surrounding normal liver kept shrinking gradually for more than a year (C). Ascites appeared six months after the proton irradiation, and its volume increased gradually (C). At 12 months after the proton beam therapy, multiple lung metastases developed, but the liver tumor was controlled. The patient started oral tegafur-uracil (UFT-E™, Taiho Pharmaceutical Co., Tokyo, Japan) chemotherapy.
Case 2
A 68-year-old female with chronic hepatitis C virus infection and Child-Pugh grade C cirrhosis received proton beam therapy in April 2002 for her solitary HCC lesion of 16-mm diameter in liver S6 (A). The pre-treatment serum AFP level was 752 ng/mL. Proton beam therapy was carried out in 60 Gy in 10 fractions over 15 days (B).
Figure 2. CT images of Case 2. (A) Pre-treatment CT image revealed a solitary nodule with a diameter of 16 mm in the liver S6 with an enhancement in the arterial phase. (B) Dose distribution of the proton beam therapy. A metallic marker was inserted near the tumor by an ultrasound-guided percutaneous procedure preceding the therapy. (C) The CT image of the arterial phase at 9 months after proton beam therapy. The tumor was locally controlled, and radiation hepatitis existed persistently. In addition, the shape of the liver around the irradiated volume became irregular because of local atrophic change in the irradiated region.
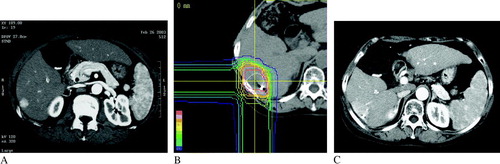
CT images showed an enhanced pattern in the irradiated normal liver tissue similar to that shown in Case 1, and the tumoral early enhancement was diminished six months after proton beam therapy. The irradiated normal liver tissue became atrophic with no obvious tumor stain remained nine months after proton beam therapy (C). At the last follow-up at 15 months after the proton beam therapy, the patient was alive and showed no apparent symptoms of hepatic failure, and no local, intrahepatic, regional, or distant recurrence was seen.
Case 3
A 62-year-old male with multiple metastatic liver tumors from rectal cancer visited us on April 2003 to receive proton beam therapy for the liver tumors. He was negative for both the hepatitis B and C viruses. The primary tumor was controlled after low anterior resection in August 2002. Liver tumors grew in spite of post-operative systemic chemotherapy with 5-fluorouracil and leucovorin. On his first visit, CT showed the number of liver tumors to be five (A). The three larger tumors (64 mm, 60 mm, and 30 mm in diameter in S4, S6, and S8, respectively) were targeted for proton irradiation with palliative intent. He received 66 Gy, 60 Gy, and 60 Gy of proton beam therapy in 22, 10, and 10 fractions over 32, 12, and 17 days, respectively (B).
Figure 3. CT images of Case 3. (A) Pre-treatment enhanced CT image with multiple metastatic tumors. (B) Dose distributions of the proton beam therapy for the largest three tumors. (C) The enhanced CT image at one month after proton beam therapy. The density of the irradiated liver tissue was low, unlike in Cases 1 and 2. This represented a different enhancement pattern of the radiation hepatitis Citation[15].
![Figure 3. CT images of Case 3. (A) Pre-treatment enhanced CT image with multiple metastatic tumors. (B) Dose distributions of the proton beam therapy for the largest three tumors. (C) The enhanced CT image at one month after proton beam therapy. The density of the irradiated liver tissue was low, unlike in Cases 1 and 2. This represented a different enhancement pattern of the radiation hepatitis Citation[15].](/cms/asset/bf748bdc-3cd5-4e34-a118-3d15b13b89ea/ionc_a_169003_f0003_b.jpg)
The irradiated normal liver tissue around the tumors had low density on enhanced CT one month after the proton irradiation (C). At 12 months after the proton beam therapy, multiple lung metastases developed, and the patient started oral fluorinated pyrimidine (S-1™, Taiho Pharmaceutical Co., Tokyo, Japan) chemotherapy. He was alive with no evidence of regrowth in the liver tumors at 16 months after the proton beam therapy. No clinical symptoms associated with hepatic failure were observed.
Sequential evaluation by 99mTc-GSA scintigraphy and blood tests
Hepatic functional reserves in all three patients were sequentially evaluated by 99mTc-GSA scintigraphy and blood tests, before and at the end of a series of proton irradiation, and at several months’ intervals up to more than a year after proton beam therapy. All three patients exhibited similar changes in uptake pattern as described below. Single photon emission computed tomography (SPECT) images of 99mTc-GSA scintigraphy for Case 1 were sequentially exhibited (D–1G).
SPECT images revealed focal uptake defects corresponding with the liver tumors before proton beam therapy (D). At the end of proton beam therapy, the defect regions were extended (E), which reflected early radiation damage of the normal liver tissue around the tumor. In Case 1, 99mTc-GSA uptake in the left lobe of the liver relatively increased, because of the large uptake defect in the right lobe (E). Thereafter, the uptake defects were consistently observed more than 12 months after the proton beam therapy (F and 1G). The transient enlargement of the higher uptake area (red area of the SPECT images) in F appeared to reflect compensatory hypertrophy of the left lobe.
The ratio of liver activity to heart and liver activity at 15 minutes (LHL15) was stable at the end of the proton beam therapy ( and ). But the LHL15 value transiently declined three to six months after the proton beam therapy, and it recovered to the pre-treatment level in about one year. Other blood count and chemical analysis data had no distinctive changes in all three cases except in the tumor marker level. The time courses of the blood analysis data are shown in .
Table I. The time-course of the liver function index and laboratory data in Case 1.
Table II. The time-course of LHL15 index in Cases 2 and 3.
Discussion
In the present study, we report sequential changes in hepatic functional reserve assessed by 99mTc-GSA scintigraphy before and after proton beam therapy delivered to liver tumors in three patients. In these three patients, the LHL15 index, which is a reproducible index of hepatic functional reserve even in cases of hepatic dysfunction and correlates well with classical indicators for hepatic function Citation[14], Citation[16], Citation[17], deteriorated transiently 3–6 months after the proton beam therapy, and recovered nearly to the pre-treatment level in a year. The time course of the calculated LHL15 value was not consistent with the regenerating functional normal liver tissue volume or blood analyses.
Concerning the post-hepatectomy hepatic functional reserve as assessed by 99mTc-GSA scintigraphy, higher LHL15 values compared with those expected from the remaining normal liver tissue volume have been observed soon after the surgery in other series Citation[18], Citation[19]. These observations seem to contradict the intact hepatocyte theory Citation[20], which presumed that the asialoglycoprotein receptor (ASGPR) expression was proportional to the number of normal hepatocytes, irrespective of the severity of the liver cirrhosis. The discrepancy between the normal liver tissue volume and the assumed number of the ASGPR can be explained by either increased ASGPR expression per hepatocyte or an ASGPR affinity change induced by hepatectomy.
Clinically, hepatic regeneration is induced by hepatectomy or hepatic irradiation Citation[15], Citation[21], Citation[22]. Up-regulation of hepatocyte growth factor (HGF) expression is observed soon after hepatectomy Citation[23], Citation[24] or irradiation Citation[25]. HGF protein expression in the liver then decreases to the normal level within ten days after hepatectomy Citation[24] or within a month after irradiation Citation[25]. Shortly after hepatectomy, normal liver tissue volume has not yet increased because hepatic regeneration is not fully achieved, but the LHL15 value is not much different from the pre-operative one Citation[18], Citation[19]. This phenomenon is explained by the HGF-driven functional up-regulation of ASGPR. Actually, increased uptake of 99mTc-GSA per hepatocyte was observed to result from recombinant HGF administration Citation[26].
There have been few studies involving the results of 99mTc-GSA scintigraphy after irradiation Citation[27]. In our observation, 99mTc-GSA uptake in the peri-tumoral normal liver tissue decreased at the end of the proton beam therapy in all three patients. This finding meant that the normal hepatocytes were deprived of their function soon after the proton irradiation. Moreover, the LHL15 index fell later, and recovered nearly to the pre-treatment level within a year ( and ), although hepatic regeneration has been seen to continue morphologically more than six years after proton beam therapy Citation[15]. The discrepancy in the time course between the expected hepatic functional reserve and the LHL15 index was similar to that observed after hepatectomy Citation[19].
Conclusions
We report the distinctive course of the hepatic functional reserve as evaluated by 99mTc-GSA scintigraphy in three patients with liver tumors treated by proton beam therapy. The LHL15 index, which is known to be an alternative indicator of hepatic functional reserve, deteriorated transiently 3–6 months after proton beam therapy, and recovered in one year. The time course was not consistent with the normal liver tissue volume or blood analyses. This might be related to HGF-driven liver regeneration. Further investigations involving co-ordinated analyses of blood tests, CT, and 99mTc-GSA scintigraphy during and after the proton beam therapy are needed to provide a detailed description of the relationship between irradiation and the hepatic functional reserve in patients with liver tumors.
References
- Nishioka K, Watanabe J, Furuta S, Tanaka E, Iino S, Suzuki H, et al. A high prevalence of antibody to the hepatitis C virus in patients with hepatocellular carcinoma in Japan. Cancer 1991; 67: 429–33
- Tanaka K, Hirohata T, Koga S, Sugimachi K, Kanematsu T, Ohryohji F, et al. Hepatitis C and hepatitis B in the etiology of hepatocellular carcinoma in the Japanese population. Cancer Res 1991; 51: 2842–7
- Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg 1995; 19: 59–71
- Kokudo N, Imamura H, Sugawara Y, Sakamoto Y, Yamamoto J, Seki M, et al. Surgery for multiple hepatic colorectal metastases. J Hepatobiliary Pancreat Surg 2004; 11: 84–91
- Suit H, Goldberg S, Niemierko A, Trofimov A, Adams J, Paganetti H, et al. Proton beams to replace photon beams in radical dose treatments. Acta Oncol 2003; 42: 800–8
- Brahme A. Recent advances in light ion radiation therapy. Int J Radiat Oncol Biol Phys 2004; 58: 603–16
- Bentzen SM. Steepness of the radiation dose-response curve for dose-per-fraction escalation keeping the number of fractions fixed. Acta Oncol 2005; 44: 825–8
- Glimelius B, Ask A, Bjelkengren G, Bjork-Eriksson T, Blomquist E, Johansson B, et al. Number of patients potentially eligible for proton therapy. Acta Oncol 2005; 44: 836–49
- Chiba T, Tokuuye K, Matsuzaki Y, Sugahara S, Chuganji Y, Kagei K, et al. Proton beam therapy for hepatocellular carcinoma: A retrospective review of 162 patients. Clin Cancer Res 2005; 11: 3799–805
- Hata M, Tokuuye K, Sugahara S, Kagei K, Igaki H, Hashimoto T, et al. Proton beam therapy for hepatocellular carcinoma with portal vein tumor thrombus. Cancer 2005; 104: 794–801
- Nemoto K, Tokuue K, Onishi K, Mizumoto M, Hashimoto T, Igaki H, et al. Proton beam therapy for large hepatocellular carcinoma [in Japanese]. J Jpn Soc Ther Radiol Oncol 2004; 16: 177–82
- Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol 2005; 23: 1839–46
- Bush DA, Hillebrand DJ, Slater JM, Slater JD. High-dose proton beam radiotherapy of hepatocellular carcinoma: Preliminary results of a phase II trial. Gastroenterology 2004; 127(5 Suppl 1)S189–S193
- Kudo M, Todo A, Ikekubo K, Hino M, Yonekura Y, Yamamoto K, et al. Functional hepatic imaging with receptor-binding radiopharmaceutical: Clinical potential as a measure of functioning hepatocyte mass. Gastroenterol Jpn 1991; 26: 734–41
- Ahmadi T, Itai Y, Onaya H, Yoshioka H, Okumura T, Akine Y. CT evaluation of hepatic injury following proton beam irradiation: Appearance, enhancement, and 3D size reduction pattern. J Comput Assist Tomogr 1999; 23: 655–63
- Kudo M, Todo A, Ikekubo K, Hino M. Receptor index via hepatic asialoglycoprotein receptor imaging: Correlation with chronic hepatocellular damage. Am J Gastroenterol 1992; 87: 865–70
- Wu J, Ishikawa N, Takeda T, Sato M, Fukunaga K, Todoroki T, et al. Evaluation of reserved hepatic function in patients with hepatobiliary tumor by 99mTc-GSA: Effect of hyperbilirubinemia and usefulness of regional reserved hepatic functional imaging. Kaku Igaku (Jpn J Nucl Med) 1996; 33: 161–8
- Toyama H, Ito K, Komori Y, Sugioka A, Shibata K, Komai S, et al. Evaluation of the residual functional reserve and the early regeneration after the hepatic resection using asialoglycoprotein receptor imaging agent. Kaku Igaku (Jpn J Nucl Med) 1995; 32: 323–9
- Kondo M. Usefulness of 99mTc-GSA scintigraphy for estimation of residual hepatic functions and postoperative changes of HH15 and LHL15. Kaku Igaku (Jpn J Nucl Med) 2001; 38: 191–200
- Wood AJ, Villeneuve JP, Branch RA, Rogers LW, Shand DG. Intact hepatocyte theory of impaired drug metabolism in experimental cirrhosis in the rat. Gastroenterology 1979; 76: 1358–62
- Nagasue N, Yukaya H, Ogawa Y, Kohno H, Nakamura T. Human liver regeneration after major hepatic resection. A study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg 1987; 206: 30–9
- Yamanaka N, Okamoto E, Kawamura E, Kato T, Oriyama T, Fujimoto J, et al. Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatology 1993; 18: 79–85
- Efimova EA, Glanemann M, Nussler AK, Schumacher G, Settmacher U, Jonas S, et al. Changes in serum levels of growth factors in healthy individuals after living related liver donation. Transplant Proc 2005; 37: 1074–5
- de Jong KP, von Geusau BA, Rottier CA, Bijzet J, Limburg PC, de Vries EG, et al. Serum response of hepatocyte growth factor, insulin-like growth factor-I, interleukin-6, and acute phase proteins in patients with colorectal liver metastases treated with partial hepatectomy or cryosurgery. J Hepatol 2001; 34: 422–7
- Yamazaki H, Matsumoto K, Inoue T, Nose T, Murayama S, Teshima T, et al. Induction of hepatocyte growth factor in the liver, kidney and lung following total body irradiation in rat. Cytokine 1996; 8: 927–32
- Kouda K, Ha-Kawa SK, Tanaka Y. Increased technetium-99m-GSA uptake per hepatocyte in rats with administration of dimethylnitrosamine or hepatocyte growth factor. J Nucl Med 1998; 39: 1463–7
- Fukui A, Murase K, Tsuda T, Fujii T, Ikezoe J. Assessment of liver function in chronic liver diseases and regional function of irradiated liver by means of 99mTc-galactosyl-human serum albumin liver scintigraphy and quantitative spectral analysis. Ann Nucl Med 2000; 14: 467–76