Abstract
For many years, loco-regional radiotherapy was the standard postoperative treatment for node positive breast cancer patients in Sweden. Because of encouraging results from trials of adjuvant chemotherapy in the mid 1970s, the Stockholm Breast Cancer Study Group decided to directly compare postoperative radiation (RT) with adjuvant CMF-type chemotherapy (CT). Long-term results are presented from two randomized trials of RT versus CT in pre- (n = 547) and postmenopausal (n = 679) patients, respectively, with node positive disease or a tumour diameter > 30 mm. RT substantially reduced loco-regional recurrences among both pre- and postmenopausal patients (relative hazard RT versus CT: 0.67 and 0.43, respectively). Among premenopausal patients distant metastases occurred less frequently in the CT group (relative hazard: 1.68, p > 0.001) resulting in an improved recurrence-free survival (p = 0.04). Overall survival was also better with CT (cumulative survival at 15 years: 50% and 44% in the CT and RT groups, respectively) but the difference was not statistically significant. Among the postmenopausal patients there were no substantial differences in terms of recurrence-free or overall survival between the treatment groups. The risk of a second primary malignancy, however, was doubled in the RT group (p > 0.01). The most pronounced excess concerned second lung cancers occurring after 10 years. The cumulative incidence at 20 years was estimated at 0.3% and 3.7% in the CT and RT groups, respectively. The trials illustrate the role of radiotherapy in preventing loco-regional recurrences among high-risk patients, as well as the need for systemic treatment to control the disease systemically.
In 1976 the Stockholm Breast Cancer Study Group initiated two concurrent randomized clinical trials comparing postoperative radiation therapy (RT) with adjuvant chemotherapy (CT) in pre- and postmenopausal patients, respectively. The rationale for the studies was that in the middle of the 1970s the standard treatment for high-risk, node positive patients in Stockholm was primary surgery and postoperative, loco-regional radiotherapy. Adjuvant systemic therapy was at the time still considered experimental. However, in the mid 1970s preliminary data from randomized trials indicated substantial improvements of the recurrence-free survival with adjuvant cytotoxic chemotherapy in patients with node-positive disease Citation[1], Citation[2]. Despite these promising results many clinicians were reluctant to accept cytotoxic chemotherapy as a routine treatment, particularly for older, postmenopausal patients, due to concerns related to side-effects, efficacy, and costs.
Because of these circumstances it was considered appropriate to directly compare postoperative radiotherapy with adjuvant chemotherapy to establish which should be considered the standard treatment for patients at high risk of recurrence. Today, it is widely accepted that RT can be safely administered sequentially with adjuvant CT although the optimal treatment sequence remains controversial. In the late 1970s, on the other hand, concerns about potential acute and late side effects were thought to preclude routine use of both treatment modalities in the adjuvant setting.
The postmenopausal trial also included a randomized comparison of tamoxifen (TAM) versus no adjuvant endocrine therapy. A 2×2 factorial study design was used and the patients were randomized to one of four groups: adjuvant CT, adjuvant CT plus TAM, RT, or RT plus TAM. Since the mechanism of action and the toxicity profile of TAM are different from those of the other two treatment modalities, combined treatment may result in additive effects. When the trials were initiated only little data were available on the effects of TAM in premenopausal patients so it was not considered appropriate to use the drug in that subgroup of patients.
Preliminary results from these trials were published previously Citation[3]. However, this is the first report including all 545 premenopausal and 679 postmenopausal patients entered in the trials.
Material and methods
Participating institutions
The trial was conducted by the Stockholm Breast Cancer Study Group which is a consortium including all departments of surgery and oncology involved in the care of breast cancer patients in the region. Representatives of departments of diagnostic radiology and pathology are also part of the group. The group's trial center is located at the Oncologic Centre for Stockholm-Gotland at the Karolinska Hospital, Stockholm. The trial was open for patient entry during November 1976 through May 1990.
Entry criteria
The two trials included pre- and postmenopausal women, respectively, treated with a modified radical mastectomy because of a histologically verified invasive, unilateral breast cancer. A woman was considered postmenopausal if more than six months had elapsed since her last menstrual period. After a hysterectomy a woman was considered postmenopausal if aged above 50 years. Initially the upper age limit in the postmenopausal trial was 70 years. However, in 1980 it was lowered to 65 years because of toxicity problems in the CT group.
All patients were required to have node-positive disease or a tumour diameter, measured on the surgical specimen, exceeding 30 mm. Reasons for exclusion were inoperable local disease, distant metastases at the time of primary diagnosis, other concurrent cancers, medical contraindications to the treatment, and surgery which deviated from that described in the protocol.
Hormone receptors
The clinical relevance of hormone receptor determinations in the adjuvant setting remained unclear in the mid 1970s so there was no selection of patients to the trials on the basis of receptor status. However, prospective data on estrogen receptor (ER) content were available in 486 (89%) premenopausal patients, and in 593 (87%) postmenopausal patients. All assays were done in one laboratory. Isoelectric focusing as previously described was used for all patients included in the trial before 1988 Citation[4], Citation[5]. Thereafter, this technique was replaced by a quantitative enzyme-immunoassay Citation[6], Citation[7]. The receptor values were normalized to DNA content as measured by Burton Citation[8]. There were no substantial differences between the patients with and without receptor data in regard to age, tumour size, and nodal status in either the pre- or postmenopausal trial (data not shown). A cut-off level of 0.05 fmol/microgram DNA was used to distinguish between ER positive and ER negative cases.
Sample size
The original protocol did not specify a target sample size. Instead, the trial was open until 1990, that is, until published information was available from the international overview of adjuvant trials unequivocally showing clinically worthwhile survival benefits with adjuvant CT and TAM Citation[9]. At that time, it was considered unethical to withhold systemic treatment in high-risk patients, and the trial was closed for entry.
Randomization
Randomization was by telephone to the trial centre where patient identifiers were recorded before the allocated treatment was revealed to the responsible physician. Randomization was done using balanced lists prepared with a random number table according to the permuted block technique. Within each trial the included patients were stratified according to treatment centre. No patient for whom a treatment was allocated was subsequently withdrawn from the analysis.
Among premenopausal patients the randomization was between postoperative RT and adjuvant CT. Among postmenopausal patients randomization was done using a 2×2 factorial design; the patients were randomized between RT alone, RT plus TAM, CT alone, or CT plus TAM.
Owing to a temporary shortage of radiation treatment capacity in the Stockholm area the randomization between RT and CT was deliberately unbalanced in both trials during March 1982 through May 1985: 2/3 of the patients were randomized to chemotherapy and 1/3 to radiation therapy. This explains the unbalanced number of patients included in the RT and CT groups.
Radiotherapy
Radiation therapy was initiated within 4–6 weeks of surgery. It was given with high-voltage technique. The dose was 46 Gy with 2 Gy per fraction five days a week for a total treatment time of about 4 1/2 weeks. The target volume included the chest wall, axilla, supraclavicular fossa and the ipsilateral internal mammary nodes down to the fifth intercostal space. The treatment was individually planned in all cases. Dose planning of fields covering the chest wall and internal mammary nodes was 2-dimensional and based on one cross section (the mamillary plane). The chest wall was typically irradiated with 7–14 MeV electrons and the lymph node areas with Co-60 or 4–6 MV photons.
Chemotherapy
Since 1978 the CT protocol was the same as in the first Milan trial Citation[10], that is, 12 courses of CMF (cyclophosphamide 100 mg/m2 orally on Day 1–14, methotrexate 40 mg/m2 IV on Day 1 and 8, 5-fluorouracil 600 mg/m2 IV on Day 1 and 8). However, during the first 18 months of the study cyclophosphamide was replaced by chlorambucil 10–15 mg orally on Day 1–8 and a 6-week interval between courses was allowed to avoid dose reductions. This implied that the total treatment time was up to 18 months for the 12 courses. Because of prolonged thrombocytopenia in some patients, the regimen was changed to the mentioned CMF regimen in 1978 and the cycle length was shortened to 28 days. Dose reductions were scheduled in case of hematological or gastrointestinal toxicity. Despite such reductions it was difficult to administer CT in older women owing to drug toxicity. Therefore, the upper age limit in the trial was lowered to 65 years in 1980.
In 1988, the protocol was amended to include six courses of chemotherapy instead of 12 since the overview of adjuvant chemotherapy trials did not indicate any advantage with regimens of longer versus shorter durations Citation[9].
A previous analysis of chemotherapy doses in these trials revealed that the premenopausal patients much more frequently received full protocol doses compared to the postmenopausal patients Citation[11].
Tamoxifen
TAM (40 mg daily) was initiated within 4–6 weeks of surgery, that is, concurrently with either RT or CT. The duration of treatment was two years. However, a new trial was initiated in 1983: TAM patients who were disease-free at two years were randomly allocated to stop TAM or to continue for three more years, that is, a total treatment period of five years. The details and results of this trial, which was part of a nation-wide collaboration, were reported previously Citation[12].
Follow-up
Follow-up visits were scheduled once every three months during the first two years, every six months during 2–5 years and yearly thereafter. Routine visits included a physical examination and an annual mammogram. Chest X-rays, bone scans, blood-tests, biopsies, etc. were only done if clinical signs or symptoms indicated a possible relapse. The treatment after disease recurrence was decided individually for each patient by the responsible clinician.
The vital status of all patients was checked against regional population registers and the Swedish Cause of Death Registry. All patients were also checked against the Swedish Cancer Registry.
The median follow-up was 18.4 years (range: 11–27 years). A total of 17 patients (1.4%) were lost to follow-up because of emigration.
Statistical methods
Overall and recurrence-free survival (RFS) was estimated according to the Kaplan-Meier technique Citation[13]. The end-point in calculations of RFS was any event, that is, disease recurrence, contralateral breast cancer, other cancer, or death without a reported recurrence. Loco-regional recurrence was defined as a relapse on the chest wall or in the ipsilateral regional nodes. This implied that supraclavicular recurrences were recorded as loco-regional. Patients with synchronous loco-regional and distant recurrence were considered to have had distant recurrence as their first event. The information concerning second cancer as a first event was primarily based on information supplied by the responsible clinician to the trial centre. However, this information was supplemented with data from the Swedish Cancer Registry on tumour site and histopathology. The trial protocol did not specify the clinical work-up needed to distinguish a new primary malignancy from a distant metastasis from the patient's primary breast cancer. This was left to the discretion of the responsible physician.
Crude cumulative incidence rates, that is, the failure probability for a particular type of event in the presence of other events were estimated using the Kaplan-Meier technique generalized to include competing risks Citation[14]. Survival time distributions were compared with the log-rank test Citation[15]. Hazard rate ratios (relative hazard, RH) and 95% confidence intervals (95% C. I.) were estimated using the Cox's proportional hazards model Citation[16]. Tests of interactions between treatment and various covariates were done by inclusion of product terms in the models.
Deaths among patients with a reported loco-regional and/or distant recurrence were recorded as due to breast cancer. The cause of death among other deceased patients was recorded as the underlying cause of death as recorded in the Swedish Cause of Death Registry. All randomized patients were included in the analyses irrespective of eligibility or exclusion criteria. The analyses were on the basis of intention to treat. No analyses were done on the basis of treatment received. The trial including informed consent procedures were approved by the Karolinska Institute's Regional Research Ethics Committee. Preliminary results of the trial have been published previously Citation[3], Citation[11]. However, this is the first publication including all randomized patients
Results
Patient characteristics were balanced between the RT and CT groups among both the 547 premenopausal and 679 postmenopausal patients (). Also, the distribution of nodal involvement and tumor size were about the same in the pre- and postmenopausal trial. However, as expected, the proportion of estrogen receptor positive tumors was higher among the postmenopausal than the premenopausal patients.
Table I. Patient characteristics by allocated treatment
The median number of nodes identified by the pathologist in the axillary specimen was eight among both the pre- and postmenopausal patients. The respective percentage of patients in the two trials with less than five examined nodes was 15 and 16.
Premenopausal trial
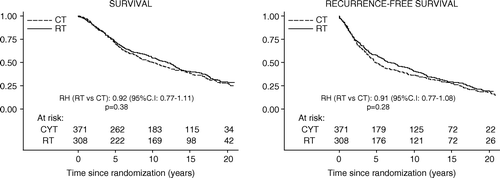
Among premenopausal patients there was a difference in favor of the RT group in terms of loco-regional recurrences: the relative hazard (RH) for RT versus the CT group was 0.67 (p > 0.05), but a substantial benefit with CT in terms of distant metastases (RH: 1.68, p > 0.001). Since distant metastases was by far the most common type of first event, and as other types of events were about equally common in the two treatment groups, there was a benefit (p = 0.04) for the CT patients in terms of RFS ().
Figure 1. Overall and recurrence-free survival in the premenopausal trial. The relative hazard (RT vs. CT group) and log rank p-value are indicated.
There were numerically fewer breast cancer deaths among patients allocated to CT corresponding to a 5% absolute reduction (RH: 1.20, ), but the difference was not statistically significant. The number of non-breast cancer deaths was too small to permit meaningful conclusions. RFS and overall survival by allocated treatment are displayed graphically in .
Table II. Analysis of events among premenopausal patients.
There was no statistically significant interaction between allocated treatment (RT vs. CT) and nodal involvement (N0, N1–3, and N4 + ) for any type of first event or cause of death, that is, the treatment effect (the relative hazard) appeared to be unrelated to nodal status (data not shown). However, because of the difference in base-line risk, the benefit in absolute terms for the RT group in terms of cumulative incidence of loco-regional recurrence at 15 years was much greater in the pN4+ subset (19 versus 34%) than among those with 1–3 involved nodes (12 versus 18%), or no involved node (7 versus 11%) ().
Table III. Cumulative incidence of events at 15 years among premenopausal patients.
Postmenopausal trial
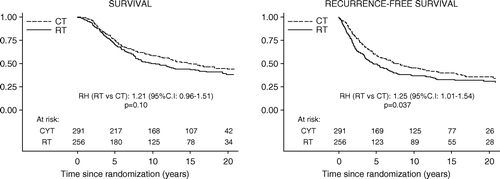
In the “main effects” analysis of RT versus CT there was a substantial benefit with RT in terms of loco-regional recurrences (RH: 0.43, p > 0.001, ). The chest wall was by far the most common site for such recurrences but the benefit with RT concerned all loco-regional subsites. Other types of events were numerically more common in the RT group but, with the exception of other cancers, there were no large differences (). The overall event rate was about the same in the RT and CT groups.
Table IV. Analysis of events among postmenopausal patients (RT versus CT).
There were numerically fewer breast cancer deaths among patients allocated to RT corresponding to a 7% absolute reduction (RH: 0.83, ), but the difference was only of borderline, statistical significance (p = 0.07). The number of non-breast cancer deaths, including cardiovascular deaths, was relatively low and about the same in the RT and CT group. Overall mortality was also about the same in the two groups. RFS and overall survival according to allocated treatment are displayed graphically in .
As in the premenopausal group, there was no evidence of interaction between the allocated treatment (RT vs. CT) and nodal involvement (N0, N1 – 3, and N4 + ) for any type of first event or cause of death, that is, the treatment effect (the relative hazard) appeared to be unrelated to nodal status (data not shown). However, there was a 15% benefit for the RT group in terms of cumulative incidence of loco-regional recurrence at 15 years in both the pN1–3 group (9 versus 25%) and pN4+ group (15 versus 30%). Among those with no involved node the estimated risk was actually slightly higher in the RT than in the CT group (12 versus 8%) but that subgroup was relatively small ().
Table V. Cumulative incidence of events at 15 years among postmenopausal patients.
The risk of second cancers other than contralateral breast cancer was more than doubled in the RT group (RH: 2.01, p = 0.010, ). An excess was observed both during the first ten years of follow-up and after ten years (). The early excess concerned a wide variety of cancer sites, whereas the excess after ten years was restricted to lung cancer (nine cases in the RT group versus none in the CT group). The respective cumulative risk of lung cancer as a first event in the RT group at 10, 15 and 20 years was 0.7%, 2.8%, and 3.7%. In the CT group there was only one case with a second lung cancer which occurred during the ninth year of follow-up. The cumulative risk in that group at 10 years was estimated at 0.3%.
Table VI. New primary malignancies other than contralateral breast cancer among postmenopausal patients by allocated treatment and period of follow-up.
Adjuvant tamoxifen
In the “main effects” analysis of TAM versus no TAM there was a statistically significant benefit with TAM in terms of events (RH: 0.79, p > 0.01), breast cancer deaths (RH: 0.74, p > 0.01), and a trend towards improved overall survival (RH: 0.84, p = 0.06) (). The relative hazard for second cancers indicated a two-fold excess in the TAM group. This excess mainly concerned endometrial cancer (data not shown).
Table VII. Analysis of events among postmenopausal patients (TAM versus no TAM).
The RFS benefit with TAM appeared to be restricted to those with ER positive tumors although the test for interaction was not statistically significant (p = 0.19). No reduction of events with TAM was observed among those classified as ER negative (RH: 0.95, 95% C. I.: 0.64–1.40, p = 0.79). In contrast, the corresponding relative hazard for those classified as ER positive was 0.69 (95% C. I.: 0.56–0.85, p > 0.001).
There was no statistically significant interaction between the effect of TAM and the effect of RT versus CT, that is, there was no evidence that the effect of RT versus CT was related to whether the patient received concurrent TAM or not ().
Table VIII. Analysis of interaction between the effect of RT/CT and TAM.
Discussion
These trials have, to some extent, a mainly historical interest since some of the treatments tested are no longer relevant to routine medical practice, for instance, adjuvant CMF and TAM duration of only two years. This is not surprising given that the trials were initiated more than 25 years ago. On the other hand, they are still of considerable value since they illustrate long-term outcomes, for instance, with regard to second cancer incidence and risk of cardiovascular disease. Long-term data on these issues inevitably concern treatments that were used a long time ago. In this regard it was nevertheless a limitation that the chemotherapy protocol was amended on several occasions: changing from chlorambucil to cyclophosphamide, increasing dose intensity by shortening the allowed treatment interval, and decreasing the total number of courses from 12 to six. By modern standards the trial recruited patients over an exceptionally long period (14 years). During this period knowledge about adjuvant cytotoxic chemotherapy increased substantially and the numerous amendments should be viewed in that light. In contrast, the radiotherapy techniques described in the protocol were left unchanged.
At the time of the trials relatively little was known about late cardiac effects of radiation. Consequently, the protocol did not specify measures to avoid excessive cardiac irradiation. Despite this circumstance we observed no increase of cardiovascular mortality in either trial associated with radiation therapy although the confidence intervals for the relative hazards were fairly wide. The typical use of electrons to treat the chest wall may help to explain this observation. An increase of cardiac mortality associated with postmastectomy radiation was reported previously Citation[17]. However, the excess of deaths due to ischemic heart disease observed in the randomized Stockholm trial of pre- or postoperative RT versus surgery alone only concerned those who received high radiation dose-volumes to the myocardium Citation[18]. Such patients were those with a left-sided breast cancer treated preoperatively with deep tangential photon fields. As in the current trial, no excess of deaths due to ischemic heart disease was observed among patients treated postoperatively with electrons to the chest wall.
In the postmenopausal trial, the increase of second cancers associated with allocation to radiotherapy during the first ten years was probably not explained by radiation-induced malignancies since such tumours typically have a latency of more than ten years. Alternative explanations are competing risks (recurrence-free survival was better for those allocated to radiotherapy during the first ten years so more event-free RT patients were at risk), or misclassification of distant metastases. However, such explanations appear less probable for the excess after 10 years, all of which concerned second lung cancers. The cumulative risk at 20 years of developing a second lung cancer as a first event was 3.7% in the RT group versus 0.3% in the CT group (p = 0.004). Our data support and extend previous information suggesting a causal relationship between postmastectomy radiation and an increased incidence of second lung cancers Citation[19]. The use of electrons to the chest wall may reduce the volumes of myocardium and lung tissue that receive high radiation doses. However, it may also be associated with relatively large lung volumes that receive intermediate or low doses of radiation, a circumstance that may have contributed to our observation. One may speculate that the excess risk of lung cancer associated with radiation was most pronounced among smokers as suggested by previous data Citation[20]. However, prospective information on smoking history was not available in the current trials. A more detailed analysis of the putative relationship between the radiotherapy and risk of lung cancer was not within the scope of this paper as it would require reconstructions of lung dose volumes in relation to the location of the cancer. This is difficult because the original dose plans were two-dimensional and based on only one cross-section through the mamillary plane. Moreover, the clinical data reported on second cancers did not include more detailed information on tumour location, and registration of lung cancer laterality has been incomplete and inconsistent in the Swedish Cancer Registry.
There was no statistically significant overall survival difference between the RT and CT groups among neither pre- nor postmenopausal women but the confidence intervals of the relative hazards in these comparisons were wide. The point estimates of the relative hazards suggested about one fifth fewer deaths with CT among the premenopausal patients (as a result of a decrease of the same magnitude in breast cancer mortality), and a marginal reduction of deaths with RT among the postmenopausal patients (as a result of a decreased breast cancer mortality being balanced by an increased mortality from non-breast cancer causes). In these respects, the current results accord with the over-views of adjuvant breast cancer trials which showed a survival benefit with CT among patients younger than 50 years corresponding to a relative reduction of about one fourth, and a benefit about half that size among those aged 50–70 years Citation[19]. In the radiotherapy overview there was a breast cancer specific survival benefit with RT corresponding to a 6% relative reduction of deaths, although this benefit was balanced by an excess of non-breast cancer deaths (mainly vascular deaths) Citation[22].
Among both pre- and postmenopausal patients RT was more effective than CT for prevention of loco-regional failures. Substantial reductions of such failures were observed both among those with one to three involved nodes, and those with four or more positive nodes. These results accord with the Danish and British Columbia randomized trials of postmastectomy radiation Citation[21–23] and underscore the relevance of combined CT and RT among women at high risk of both local and distant failure. In fact, an overview of RT in the presence of adjuvant CT suggested that the overall survival benefit associated with RT in that setting was much greater than indicated by the mentioned overview of all RT trials Citation[26]. However, routine use of loco-regional radiation among patients with one to three involved nodes remains controversial. It could be argued that with modern breast cancer surgery performed by dedicated breast surgeons, loco-regional failure rates in this subset is so low that the benefit resulting from addition of radiation therapy is only marginal and clinically not worthwhile. Our observation of a substantial benefit with radiotherapy in terms of loco-regional failures also in this subset could be related to the fact that, at the time of the trial, breast cancer surgery in the Stockholm area was performed by a large number of general surgeons at several departments of surgery. The median number of excised nodes among patients included in the current trials was eight and in 15 – 16% of the patients fewer than five nodes were retrieved by the pathologist in the axillary specimen. Another indication that the surgery may not have been optimal was the relatively high axillary recurrence rate (7%) among the non-irradiated postmenopausal patients.
In the tamoxifen comparison, the treatment benefit with tamoxifen in terms of RFS and overall survival was largely in keeping with the results of the overview of adjuvant tamoxifen trials Citation[21]. As in the overview, we observed no benefit with tamoxifen in terms of non-breast cancer deaths; in fact, the 95% confidence interval for the relative hazard (0.89–1.96) excludes a more than marginal beneficial effect of tamoxifen on non-breast cancer mortality.
There was no statistically significant interaction between the effect of TAM and chemotherapy versus RT in terms of RFS. As TAM in this trial was administered concurrently with the CT, the lack of interaction implies that we found no evidence that the CT worked less well in the presence of concurrent TAM. This result contradicts the result suggested by recent randomized trials comparing concurrent versus sequential chemo-endocrine therapy Citation[27], Citation[28], but accords with the overview which showed that the effect of chemotherapy was about the same regardless of the use of concurrent tamoxifen and vice versa Citation[21].
During the past three decades our knowledge about adjuvant therapy for breast cancer has increased dramatically. Systemic treatment with cytotoxic chemotherapy and long-term endocrine therapy with tamoxifen or aromatase inhibitors have become cornerstones of the primary management of patients with high-risk disease. The CMF chemotherapy in this trial is by many considered outdated since anthracycline-based regimens, and, more recently, the addition of taxanes, have demonstrated superior efficacy. Radiation therapy techniques have also improved, for instance, with three-dimensional treatment planning based on multiple CT slices covering the entire treatment volume and multi-leaf collimators allowing more conformal treatment. However, although these innovative strategies may be superior during short-term follow-up, and, therefore, clinically worthwhile, long term outcomes remain unknown.
In summary, this long-term follow-up of one of the early trials of adjuvant therapy for high-risk breast cancer patients illustrated the relevance of postmastectomy radiotherapy to prevent loco-regional failures both among patients with one to three, and four or more involved nodes. However, distant recurrence was the most frequent type of treatment failure. Adjuvant chemotherapy was more effective than radiotherapy in preventing such failures, at least among the premenopausal patients. We found no adverse effect from radiotherapy on long-term cardiovascular mortality, probably as a result of the typical use of electrons to treat the chest wall. On the other hand, we found an excess of second lung cancers occurring after ten years among postmenopausal patients allocated to radiotherapy. This observation supports the continued development of more conformal radiotherapy techniques aimed at reducing radiation dose-volumes in both to the myocardium and the lungs.
The study was supported by the King Gustaf V Jubilee Fund and the Swedish Cancer Society. The authors are indebted to the former and current members of the Stockholm Breast Cancer Study Group. In particular we would like to thank: Jerzy Einhorn (deceased), Arne Wallgren, Bo Nordenskjöld, Ulla Glas, Jutta Askergren, Tolle Theve, Marie-Louise Hjalmar, Anders Somell, Björn Cedermark, Lambert Skoog, Leif Perbeck. Tommy Fornander, and Sam Rotstein. We are also indebted to Ulla Johansson and Toom Signomklao for excellent data managing.
References
- Fisher B, Carbone P, Economou S G, et al. l-Phenylalanine mustard (L-PAM) in the management of primary breast cancer. A report of early findings. N Engl J Med 1975; 292: 117–22
- Bonadonna G, Brusamolino E, Valagussa P, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 1976; 294: 405–10
- Rutqvist LE, Cedermark B, Glas U, et al. Radiation therapy, chemotherapy and tamoxifen as adjuncts to surgery in early breast cancer: A summary of three randomized trials. Int J Radiat Oncol Biol Phys 1989; 16: 629–39
- Wrange Ö, Nordenskjöld B, Silfverswärd C, Granberg P-O, Gustafsson J-Å. Isoelectric focusing of estradiol receptor protein from human mammary carcinoma. A comparison to sucrose gradient analysis. Eur J Cancer 1976; 12: 695–700
- Wrange Ö, Nordenskjöld B, Gustafsson J-Å. Cytosol estradiol receptor in human mammary carcinoma. An assay based on isoelectric focusing in polyacrylamide gel. Anal Biochem 1978; 85: 416–21
- Fernö M, Borg Å, Norgren A. A comparison of two steroid receptor assays in breast cancer: Dextran-coated charcoal and isoelectric focusing. Anticancer Res 1983; 3: 243–6
- Fernö M, Borg Å, Sellberg G. Enzyme immuno assay of estrogen receptor (ER) in breast cancer biopsy samples. A comparison with isoelectric focusing and detection of ER during tamoxifen treatment. Acta Radiol Oncol 1986; 25: 171–5
- Burton KA. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of DNA. Biochem J 1956; 62: 315–22
- Early Breast Cancer Trialists′ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. N Engl J Med 1988; 319: 1681–92
- Bonadonna G, Brusamolino E, Valagussa P, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 1976; 294: 405–10
- Rutqvist LE, Cedermark B, Glas U, Johansson H, Rotstein S, et al. Randomized trial of adjuvant tamoxifen combined with postoperative radiation therapy or adjuvant chemotherapy in postmenopausal breast cancer. Cancer 1990; 66: 89–96
- Swedish Breast Cancer Cooperative Group. Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. J Natl Cancer Inst 1996; 88: 1543–9
- Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81
- Marubini E, Valsecchi MG. Competing risks. Statistics in practice: Analysing survival data from clinical trials and observational studies, V Barnett. John Wiley and Sons, Chichester 1995; 331–63
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22: 719–48
- Cox DR. Regression models and life tables. J Roy Stat Soc B 1972; 34: 187–220
- Cuzick J, Stewart H, Rutqvist LE, Houghton J, Edwards R, Redmond C, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol 1994; 12: 447–53
- Rutqvist LE, Fornander T, Lax I, Johansson H. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiat Oncol Biol Phys 1992; 22: 887–96
- Deutsch M, Land SR, Begovic M, Wieand HS, Wolmark N, Fisher B. The incidence of lung carcinoma with and without postoperative radiotherapy. Results of National Surgical Adjuvant Breast and Bowel Project (NSABP) clinical trials B-04 and B-06. Cancer 2003; 98: 1362–8
- Ford MB, Sigurdson AJ, Petruliss ES, Ng CS, Kemp B, Cooksley C, et al. Effects of smoking and radiotherapy on lung carcinoma in breast cancer survivors. Cancer 2003; 98: 1457–64
- Early Breast Cancer Trialist′s Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31 000 recurrences and 24 000 deaths among 75 000 women. Lancet 1992; 339: 1–15, 71–85
- Early Breast Cancer Trialist′s Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: An overview of the randomized trials. Lancet 2000; 355: 1757–70
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group Trial 82b Trial. N Engl J Med 1997; 337: 949–55
- Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast cancer patients given adjuvant tamoxifen. Danish Breast Cancer Cooperative Group Trial 82c Trial. Lancet 1999; 353: 1641–8
- Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 1997; 337: 956–62
- Whelan TJ, Julian J, Wright J, Jadad AR, Levine ML. Does loco-regional radiation therapy improve survival? A meta-analysis. J Clin Oncol 2000; 18: 1220–9
- Albain, K, Green, S, Ravdin, P, Cobau, C, Levine, E, Ingle, J. Adjuvant chemohormonal therapy for primary breast cancer should be sequential instead of concurrent: Initial results from intergroup trial 0100 (SWOG-8814). In:. M Perry, et al, editor. Thirty-Eighth Annual Meeting of American Society of Clinical Oncology. Am Soc Clin Onc. 2002;36a, abstract 143.
- Pico C, Martin M, Jara C, Barnadas A, Pelegri A, et al. Epirubicin-cyclophosphamide adjuvant chemotherapy plus tamoxifen administered concurrently versus sequentially: Randomized phase II trial in postmenopausal node-positive breast cancer patients. A GEICAM 9401 study. Ann Oncol 2004; 15: 79–87