Abstract
The effects of two modalities of dose-escalated radiotherapy on health-related quality of life (HRQOL) were compared. Forty-one consecutive patients were treated with a 3-D conformal (3-DC) boost to 74 Gy, and 43 with high-dose rate (HDR) brachytherapy boost (2×9 Gy), following 3-D conformal treatment to 46 Gy. Median age was 70 years in both groups, median initial PSA was 7.9 µg/l in 3-DC boost patients and 8.1 µg/l in HDR boost patients. Stage was ≤T2 in 66% and 67% and Gleason score was ≥7 in 52% and 47%, respectively. HRQOL was assessed cross-sectionally using EORTC QLQ-C30 and organ-specific PR25 modules 3 – 32 (median 19) and 4 – 25 (median 14) months after treatment, respectively. Questionnaires were completed by 93% and 97% of patients, respectively. Diarrhea and insomnia scores were significantly increased in both groups. In the PR25 module, scores of 3-DC boost and HDR boost patients for urinary, bowel and treatment-related symptoms were similar. Among responders, 34% of 3-DC boost patients and 86% of HDR boost patients had severe erectile problems. Dose escalation in prostate cancer by either 3-DC boost to 74 Gy or HDR brachytherapy boost appears to result in similar HRQOL profiles.
Escalation of radiotherapy dose beyond a conventional dose level of 70 Gy has been shown in large clinical trials to result in improved biochemical disease-free survival and overall survival in localized prostate cancer Citation[1–4]. Several techniques have been developed to deliver high doses to the prostate while sparing surrounding normal tissues, among them 3-D conformal radiotherapy, intensity-modulated radiotherapy (IMRT), photon therapy and high-dose rate (HDR) brachytherapy Citation[1–7]. Typical late toxicities of radiotherapy for prostate cancer, such as dysuria, rectal bleeding or impotence, can have a severe impact on different domains of health-related quality of life [review in Citation[8]. It is unclear how different modes of dose escalation compare with regard to quality of life. The HRQOL after dose-escalated radiotherapy for prostate cancer has recently been reported for several intensified treatment schemes, including 3-D conformal treatment to 78 Gy, external-beam radiotherapy (EBRT) followed by HDR brachytherapy boost and intensity-modulated radiotherapy (IMRT) Citation[9–13]. However, results from these reports are difficult to compare due to the choice of different HRQOL instruments, analysis of different time points and conceptual differences of treatment schedules, e.g. regarding fractionation of EBRT. Published comparative studies of HRQOL after different modalitities of dose escalation in prostate cancer are, to our knowledge, limited to one report documenting similar QoL after 3-D conformal treatment to 78 Gy and short-course hypofractionated IMRT to 70 Gy Citation[11].
Dose-escalated radiotherapy for prostate cancer might be rather new to many departments and personal experience with late toxicity is still limited for most radiation oncologists. From our own clinical experience, late toxicity and quality of life appeared similar in patients treated with HDR or 3-D conformal boost, after recovery of HDR boost patients from the second implant. In the absence of randomized trial data, we performed an exploratory cross-sectional survey of HRQOL in two comparable cohorts of consecutive patients treated at our institution. These groups had very similar baseline clinical characteristics and were treated identically with 3-D conformal radiotherapy to a dose of 46 Gy, followed by either a 3-D conformal boost to 74 Gy or an HDR brachytherapy boost (two fractions of 9 Gy).
Materials and methods
Patients
Both the 3-D conformal (3-DC) boost to a dose level of 74 Gy and HDR brachytherapy boost were introduced as methods of dose escalation in localized prostate cancer at our institution in 2001. All patients treated with curative-intent radiotherapy for prostate cancer between March 2001 and September 2003 were treated with either of the two methods and are included in the present analysis. HDR brachytherapy is performed in cooperation with the urology department of a local teaching hospital and patients referred from this department and considered suitable for dose-escalated radiotherapy were treated with HDR brachytherapy boost. Patients referred by other urologists and considered suitable for dose-escalated radiotherapy were treated with 3-D conformal therapy to 74 Gy. The clinical characteristics of the n = 41 consecutive patients treated with 3-DC boost to 74 Gy and the n = 43 consecutive patients treated with two HDR boost fractions after 46 Gy are shown in .
Table I. Clinical and treatment characteristics of patients with localized prostate cancer treated with either 3-D conformal (3-DC) boost to 74 Gy or high-dose rate (HDR) brachytherapy boost (two fractions of 9 Gy each), both following 46 Gy to prostate and seminal vesicles in 3-D conformal technique. Data were compared between groups by Mann-Whitney U test where applicable (EBRT: external-beam radiotherapy; n. a.: not available).
Treatment
Treatment details for both groups are also given in . Planning computed tomography (CT) in the supine position was performed in all patients. No fixation devices or rectal balloon were applied. The region of the prostate was scanned at 5 mm slice thickness; the remaining pelvis at 10 mm. Treatment was planned using Helax-TMS 6.1 software with pencil-beam algorithm (Nucletron, Veenendal, Netherlands). In all patients, the planning target volume (PTV) encompassed the prostate and seminal vesicles, but not the pelvic lymphatics. Defined margins around the prostate and seminal vesicles were not used. As a rule, the dorsal PTV border was contoured such that the lateral beams would not include more than half of the rectal circumference. All patients were treated by a 3-D conformal four-field box technique using 18 MV photons from a Siemens (Concord/CA, USA) Primus accelerator with multi-leaf collimation. In some patients, 6 MV photons were used for the anterior field with the goal of reducing rectal dose. Typical weighting for beams from 0°/180°/90°/270° was 22%/16%/31%/31% (relative monitor units).
Fraction size for EBRT was 2 Gy. After 46 Gy, in the 3-DC boost group external beam treatment of the same PTV was continued to a dose of 60 Gy via a 3-field technique (0°/100°/260°) with strong weighting of lateral beams (typically 30%/35%/35%) with the goal of reducing dose to the posterior rectal wall. After 60 Gy a boost PTV, encompassing the prostate only without seminal vesicles, was treated to 74 Gy with a field-shape-adapted 3-field technique using identical angles and weights. In 11 of 41 (27%) patients, the volume was reduced a second time after 66 Gy or 70 Gy, at the discretion of the treating physician.
In the HDR boost group, brachytherapy was typically initiated after an interval of at least two weeks from completion of EBRT. HDR brachytherapy was delivered following the technique of the Kiel group Citation[1]. Briefly, parallel catheters were implanted transperineally via a template into the prostate of the anaesthesized patient in the lithotomy position. Catheters were arranged peripherally in the prostate in horseshoe shape under visualization by a transrectal ultrasound probe attached to a stepping unit acquiring images at 5-mm steps. Treatment was planned using in-house software and Nucletron Plato software. High-dose rate brachytherapy boost was delivered by Nucletron MicroSelectron afterloading system with iridium 192. Needle loading was optimized to achieve a dose of 9 Gy per fraction encompassing the prostate without the seminal vesicles. A margin was not routinely used, but sufficient coverage of the capsule considered in patients at high risk for capsule invasion. A second goal of plan optimization was treatment of the peripheral zone of the prostate according to Mc Neal Citation[14], with sparing of the urethra, to a fraction dose of 15 Gy (“boost-in-boost” concept). In a typical implant, the Dmax of the urethra and rectum were 13 Gy and 9 Gy, respectively. V15Gy, the percentage of prostate volume receiving at least 15 Gy, was 50%. A second HDR brachytherapy boost fraction was typically applied after another two-week interval.
Data collection
In this cross-sectional survey, all patients were mailed in December of 2003 the HRQOL questionnaires with an accompanying letter explaining the purpose of the survey, the voluntary nature of participation, the confidential processing of data, and with a postage-paid envelope. A returned questionnaire was considered as written informed consent. Patients were not contacted again if they failed to respond. Of n = 41 consecutive patients treated with 3-DC boost, n = 40 patients were contacted. One patient had died from metastatic prostate cancer. Of n = 43 consecutive patients treated with HDR boost, n = 39 were contacted. Two patients had died from causes unrelated to prostate cancer. One patient could not be located and one patient was not expected to be capable of completing the questionnaires due to mental retardation. There had been no evidence of a progression of prostate cancer in any of these four patients until the time of analysis. Clinical data were retrieved from radiotherapy and urology charts.
HRQOL instruments
The instruments used were the generic questionnaire EORTC QLQ-C30 (version 3.0) and the prostate organ module PR25 (kindly provided by the EORTC Quality of Life Study Group) Citation[15]. Both instruments were developed by the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Study Group for measuring the quality of life of cancer patients in clinical trials. The QLQ-C30 contains 30 items and covers the domains of physical, role, emotional, cognitive, and social function as well as global health status (multi-item scales). In addition, it covers several specific physical symptoms (fatigue, nausea and vomiting, pain, dyspnoea, sleep disturbance, loss of appetite, constipation, and diarrhea) as well as financial difficulties. Each item is scored from 1 to 4 (“not at all”: 1; “a little”: 2; “quite a bit”: 3; “very much”: 4). As an exception, global quality of life is scored from 1 (“very poor”) to 7 (“excellent”). Normative data for the QLQ-C30 are available for different populations. In the context of the present study, published reference data for the general German population were used for comparison Citation[16].
The PR25 questionnaire is a prostate-specific module to be used in conjunction with QLQ-C30 which contains 25 items, covering the multi-item scales of urinary symptoms (nine items), bowel symptoms (four items), treatment-related symptoms (six items) and sexual functioning (six items) Citation[17].
Questionnaire data were processed according to the procedures outlined in the EORTC QLQ-C30 scoring manual Citation[18]. Raw scores were transformed to a scale from 0 to 100. The data from the prostate module PR25 were treated accordingly, using the provisional scale structure suggested by the EORTC Quality of Life Study Group. Missing item data was treated according to the recommendations given in the manual. If at least half of the values for one scale were available in an individual patient, only these values were considered in transformation (essentially assuming that this patient's score for the missing items would equal the average of his scores for other items on the same scale). For functional scales and global quality of life, high scores represent good functioning/good quality of life. For the symptom scales, high scores indicate severe symptoms. To facilitate comparison of PR25 data with data from a previous publication using this module in patients treated with HDR brachytherapy boost Citation[13], results for single items from PR25 are also given separately.
Statistical analysis
Cronbach's coefficient α was calculated to determine internal consistency of scales within the PR25 module. HRQOL scores, as well as patient and treatment characteristics (where applicable), were compared between treatment groups using the Mann-Whitney U test. For comparison of the results of surveyed groups to the German general population, published data for the general population Citation[16] were adjusted for sex and age according to method proposed by Hjermstad et al. Citation[19], Citation[20] to create a data set of “expected values” for a normal group with the age characteristics of the surveyed groups. In principle, the number of surveyed patients per age group (50 – 59, 69 – 69, 70 and older) was multiplied with the respective mean score for the QoL category and the sum for all age groups divided by the patient number. As suggested by Hjermstad et al. Citation[19], Citation[20], results for surveyed groups were compared to this expected mean using simple t-test and assuming a standard error of 0.5 for the expected mean. To take into account multiple comparisons, differences between groups were considered significant at a level of p < 0.01. Indicated p-values are from two-tailed tests. Analysis was performed using Statistica version 6 (Statsoft, Tulsa, OK, USA) software.
Results
The response rates were 93% and 97% in the 3-DC boost and HDR boost groups, respectively. The mean time interval between end of radiotherapy and questionnaire completion was 14 months (range 4 – 25 months) in the HDR boost group and 19 months (3 – 32 months) in the 3-DC boost group. At the time of HRQOL detection, 21% of patients in the 3-DC boost group and 17% of patients in the HDR boost group had a most recent PSA value of > 1 µg/ml. However, due to limited follow-up in some patients where nadir had not yet been reached, this could not be used as a likely indicator of recurrence. Of the patients contacted, one in each group had developed bone metastases; one additional patient in the 3-DC group had a local recurrence. Twenty two percent of HDR boost patients and 12% of 3-DC boost patients had received some form of hormonal therapy within six months of questionnaire administration.
As the full psychometric properties of the module PR25 have not yet been published, internal consistency was calculated for the scales of this module. Cronbach's coefficient α was 0.88 for urinary symptoms (after exclusion of item no. 38 referring to discomfort due to use of pads, a question completed only by 10/37 and 4/38 responders in the 3-DC boost and HDR boost groups, respectively), 0.71 for bowel symptoms and 0.54 for treatment-related symptoms. Due to poor compliance for sexual functioning, α was not calculated for this scale.
The results of EORTC QLQ-C30 and PR25 questionnaires are given in . Sufficient data for each scale were available for at least 34/37 and 36/38 responders treated with 3-DC boost and HDR boost, respectively. An exception was the sexual function scale of the PR25 module for which data was available only in 16/37 and 16/38 patients, respectively. Data for this scale was therefore excluded and presented as single item scores.
Table II. Results of EORTC QLQ-C30 and of the prostate module PR25 in patients treated with either high-dose rate brachytherapy boost (HDR boost) or 3-D conformal boost to 74 Gy (3-DC boost). On function and global quality of life scales, higher scores indicate better function or better QoL. On symptom scales (QLQ-C30 and PR25) higher scores indicate more severe symptoms.
No significant differences between patients treated with 3-DC boost or HDR boost were detected in any of the scales of QLQ-C30 or PR25 (). Data for treated groups were compared to age-adjusted data for the general male German population, which is available for QLQ-C30 only Citation[16] (). Within the functional scales, the only significant difference was seen for cognitive function where the 3-DC boost group had worse scores than the general population (p < 0.01). For both treatment groups, significantly increased symptom scores for insomnia and diarrhea were seen. Analysis of response to selected single items from PR25, as performed in a previous study following HDR brachytherapy boost Citation[13], revealed that among responders erectile problems were more frequent and severe in the HDR brachytherapy boost group than in 3-DC boost patients ().
Figure 1. Results of EORTC QLQ-C30 in patients treated with either high-dose rate brachytherapy boost (HDR boost, n = 38 responders) or 3-D conformal boost to 74 Gy (3-DC boost, n = 37 responders), compared to age-adjusted scores of the German general population (see text). (A) Mean scores on function scales and global quality of life (QoL), higher scores indicating better function, better QoL. (B) Mean scores on symptom scales and single items, higher scores indicating more severe symptoms.
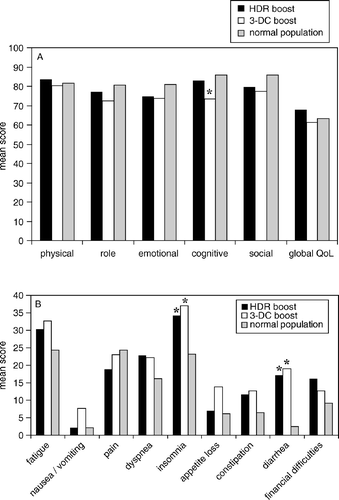
Table III. Results of individual items of the prostate module PR25 in patients treated with either high-dose rate brachytherapy boost (HDR boost) or 3-D conformal boost to 74 Gy (3-DC boost). Percentages by symptom severity, as scored from 1 to 4 (1 = “not at all”, 2 = “a little”, 3 = “quite a bit”, 4 = “very much”), are given. Mean scores for individual items were compared between groups by Mann-Whitney U test. Questions regarding sexual function were only answered by a minority of patients in both groups.
Discussion
Radical radiotherapy of localized prostate cancer with 70 Gy or less is now regarded as suboptimal for most patients and dose escalation beyond 70 Gy can be achieved by different techniques Citation[21], Citation[22]. Mature results from a randomized trial, however, have raised the concern that dose escalation might be detrimental to HRQOL Citation[2].
Due to referral patterns and institutional policy, similar patient cohorts were treated with either 3-D conformal (3-DC) boost to 74 Gy or two fractions of high-dose rate (HDR) boost of 9 Gy each. In both groups, identical 3-D conformal treatment of prostate and seminal vesicles was performed to a dose of 46 Gy. In a cross-sectional survey of HRQOL 3 to 32 months (median 19 months) and 4 to 25 months (median 14 months) after 3-DC boost and HDR boost, respectively, patients completed the questionnaire EORTC QLQ-C30 and the organ-specific module PR25.
In the two groups well balanced by prognostic factors, no significant differences in any functional or symptom domain of QLQ-C30 were observed. As a whole, the functional results for both groups, including global QoL, were quite comparable with age-adjusted results for the male general population, with the exception of reduced cognitive function in the 3-DC boost group. This effect is not explainable by any known side effect of radiotherapy. On the symptom scales of insomnia and diarrhea, both radiotherapy cohorts had significantly elevated scores, compared to the general population. Both groups scored at least about 10 points higher than controls in these categories, indicating increased symptoms at a clinically meaningful level Citation[23].
For the prostate module PR25, full psychometric properties are not yet available. Calculation of Cronbach's coefficient α to determine internal consistency of the hypothesized scales revealed several problems. One question in the urinary symptoms scale, referring to discomfort caused by wearing of pads, was not answered by the majority of patients. Since all these cases would have to be excluded from calculation of α as “missing data”, we decided to eliminate this item from the scale. The calculated α value for this scale and for bowel symptoms was above the level of 0.7 required to call a scale internally consistent. The value for treatment-related symptoms was insufficiently low at 0.54. This was not unexpected, as questions from this scale relate to side effects of hormonal treatment such as gynaecomastia, hot flushes, weight gain and ankle edema. Compliance with questions regarding sexuality was low as in a previous study using the PR25 module Citation[13]. Therefore, α was not calculated for this scale. Cronbach's α for all scales of the PR25 module has not been previously published for the PR25 module to the authors’ knowledge. One study in advanced prostate cancer patients reported a value for α of 0.86 for the urinary symptoms scale, but no results for other scales suggesting values were lower Citation[24].
On the scales of urinary, bowel and treatment-related symptoms, again no significant differences were seen between 3-DC boost and HDR boost patients. To compare our results to published data obtained after HDR brachytherapy boost using the PR25 module Citation[13] and to obtain some information on sexual function, we analyzed selected items from PR25. For questions relating to specific urinary symptoms, fecal incontinence and bleeding, no difference between radiotherapy groups was seen. Scores for sexual interest were also similar, but, among responders, severe erectile problems were significantly more frequent in the HDR group. The low response rate for this particular question can have two reasons. Either patients in general feel especially uncomfortable answering a question regarding erectile function or those having problems are less likely to respond. In the first case, the responders might be largely representative of their overall treatment groups, in the latter, non-responders would be expected to have rather poor erectile function. In both scenarios, the percentage of patients reporting “not at all” or “a little” problems with erectile function is about three times as high in the 3-DC boost group as in the HDR boost group.
A previous report on prostate-specific HRQOL measured with the PR25 module described results of a Swedish investigation at different time points after a split-course scheme of 50 Gy external beam therapy to the prostate and seminal vesicles with two fractions of HDR brachytherapy of 10 Gy each, ten days apart, after 25 Gy Citation[13]. Single item data was reported for 93 responders with a median age of 68 years. In recurrence-free patients six to 18 months after treatment, for instance, percentages of patients reporting “quite a bit” or “very much” symptoms were 40% for urgency, 12% for faecal blood, and 63% for erectile problems. In our series, the corresponding rates were 63% for urgency, 14% for fecal blood and 86% for erectile problems after HDR boost and 63% for urgency, 9% for fecal blood and 44% for erectile problems after 3-DC boost.
In the M. D. Anderson randomized trial of 78 vs. 70 Gy of 3-D conformal therapy, HQROL data after three years was available for 35 patients in each arm, corresponding to a 69% response rate Citation[12]. On a 24-item symptom questionnaire, no significant differences between treatment groups were detected. Rates of dysuria (“occasional” or “frequent”) were 23% in both groups, the percentage reporting faecal blood as “once a week” or “daily” was 9% in both groups. Full or partial erections were possible in 62% in the 78-Gy group and 78% in the 70-Gy group.
In another report, patients treated with 78 Gy of 3-D conformal treatment were compared to a cohort irradiated with 70 Gy of hypofractionated IMRT at single doses of 2.5 Gy which the authors considered to be similarly biologically effective Citation[11]. Twenty-four IMRT patients and 46 patient treated to 78 Gy completed the Expanded Prostate Cancer Index Composite about two years after treatment. Similar urinary and bowel summary scores were found. Poorer sexual summary scores in the 78 Gy 3-D conformal group were attributed to the higher proportion of patients receiving adjuvant androgen deprivation.
The Kiel group recently reported results of QLQ-C30 and a newly developed 22-item prostate module 6.5 years (mean) after whole-pelvis radiotherapy (50 Gy, prostate dose limited to 40 Gy) followed by HDR brachytherapy boost (two sessions of 8 to 9 Gy to the prostate) Citation[10]. QLQ-C30 results, as taken from their graph, appear to be equal or worse in virtually all function and symptom scales than now reported for 3-DC boost and HDR boost patients.
A Japanese group assessed HRQOL prospectively with the SF-36 questionnaire and self-formulated prostate-specific questions during the first 18 months after treatment with five times 4 to 5 Gy of HDR brachytherapy followed by ten 3-Gy fractions of EBRT to the prostate and seminal vesicles Citation[9]. While significant deterioration was seen in five domains of SF-36 at one month after radiotherapy, these values returned to normal by 12 months.
The interpretability of the data presented is limited by three main factors. First, due to the cross-sectional design, the lack of baseline QoL data, and the possibility of bias in patient selection for either treatment method, it cannot be excluded that the two patient groups had significant differences in their QoL profiles before treatment. However, the documented distribution of risk factors and age suggests that the two surveyed groups were in fact comparable. Second, the cross-sectional survey at one time point for both groups resulted in slightly different follow-up times between groups as well as heterogeneity within groups. It can be concluded from literature data that late gastrointestinal and genitourinary effects as well as effects on potency develop over a period of at least three to five years after radiotherapy for prostate cancer before reaching a plateau Citation[25]. Therefore, this cross-sectional analysis can only represent a snapshot of QoL profiles. Third, the low patient numbers analyzed make the presence of a type II error possible and differences between dose escalation methods may become apparent with larger cohorts.
In summary, our data generate the hypothesis that a 3-DC boost to 74 Gy and HDR brachytherapy boost in comparable groups of patients with localized prostate cancer result in comparable QoL. Very high biologically equivalent doses near and above 200 Gy have been calculated for HDR boost schedules Citation[26] and recurrence rates after HDR boost treatment are comparable to the highest-dose IMRT series Citation[1], Citation[4]. HDR brachytherapy boost and 3-D conformal treatment to 76 Gy are under evaluation in a recently initiated randomized trial in Germany in which QoL will be studied as a secondary endpoint Citation[27].
References
- Galalae RM, Martinez A, Mate T, Mitchell C, Edmundsun G, Nuernberg N, et al. Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. Int J Radiat Oncol Biol Phys 2004; 58: 1048–55
- Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, Huang E, et al. Prostate cancer radiation dose response: Results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 2002; 53: 1097–105
- Pollack A, Hanlon AL, Horwitz EM, Feigenberg SJ, Uzzo RG, Hanks GE. Prostate cancer radiotherapy dose response: An update of the Fox Chase experience. J Urol 2004; 171: 1132–6
- Zelefsky MJ, Fuks Z, Hunt M, Yamada Y, Marion C, Ling CC, et al. High-dose intensity modulated radiation therapy for prostate cancer: Early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys 2002; 53: 1111–6
- Aebersold DM, Isaak B, Thalmann G, Behrensmeier F, Kolotas C, Kranzbuhler H, et al. Applicability and dosimetric impact of ultrasound-based preplanning in high-dose rate brachytherapy of prostate cancer. Strahlenther Onkol 2004; 180: 351–7
- Martin T, Baltas D, Kurek R, Roddiger S, Kontova M, Anagnostopoulos G, et al. 3-D conformal HDR brachytherapy as monotherapy for localized prostate cancer: A pilot study. Strahlenther Onkol 2004; 180: 225–32
- Wahlgren T, Nilsson S, Ryberg M, Lennernas B, Brandberg Y. Combined curative radiotherapy including HDR brachytherapy and androgen deprivation in localised prostate cancer: A prospective assessment of acute and late treatment toxicity. Acta Oncol 2005; 44: 633–43
- Penson DF, Litwin MS, Aaronson NK. Health related quality of life in men with prostate cancer. J Urol 2003; 169: 1653–61
- Egawa S, Shimura S, Irie A, Kitano M, Nishiguchi I, Kuwao S, et al. Toxicity and health-related quality of life during and after high-dose rate brachytherapy followed by external beam radiotherapy for prostate cancer. Jpn J Clin Oncol 2001; 31: 541–7
- Galalae RM, Loch T, Riemer B, Rhezak P, Kuchler T, Kimmig B, et al. Health-related quality of life measurement in long-term survivors and outcome following radical radiotherapy for localized prostate cancer. Strahlenther Onkol 2004; 180: 582–9
- Kupelian PA, Reddy CA, Klein EA, Willoughby TR. Short-course intensity modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Preliminary results on late toxicity and quality of life. Int J Radiat Oncol Biol Phys 2001; 51: 988–93
- Little DJ, Kuban DA, Levy LB, Zagars GK, Pollack A. Quality-of-life questionnaire results 2 and 3 years after radiotherapy for prostate cancer in a randomized dose escalastion study. Urology 2003; 62: 707–13
- Wahlgren T, Brandberg Y, Häggarth L, Hellström M, Nilsson S. Health-related quality of life in men after treatment of localized prostate cancer with external beam radiotherapy combined with 192Ir brachytherapy: A prospective study of 93 cases using the EORTC questionnaires QLQ-C30 and QLQ-PR25. Int J Radiat Oncol Biol Phys 2004; 60: 51–9
- Mc Neal JF. The zonal anatomy of the prostate. Prostate 1981; 2: 35–49
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–76
- Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer 2001; 37: 1345–51
- Aaronson NK, van Andel G. An international field study of the reliability and validity of the QLQ-C30 and a disease-specific questionnaire module (QLQ-PR25) for assessing quality of life of patients with prostate cancer. European Organization for Research and Treatment of Cancer study protocol 15011. Brussels: 2002.
- Fayers PM, Aaronson NK, Bjordal K, Curran D, Bottomley A, on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual. 3rd ed. European Organisation for Research and Treatment of Cancer. Brussels: 2001.
- Hjermstad MJ, Fayers PM, Bjordal K, Kaasa S. Using reference data on quality of life – the importance of adjusting for age and gender, exemplified by the EORTC QLQ-C30 (+3). Eur J Cancer 1998; 34: 1381–9
- Gulbrandsen N, Hjermstad MJ, Wisloff F, Nordic Myeloma Study Group. Interpretation of quality of life scores in multiple myeloma by comparison with a reference population and assessment of the clinical importance of score differences. Eur J Haematol 2004; 72: 172–80
- Nilsson S, Norlen BJ, Widmark A. A systematic overview of radiation therapy effects in prostate cancer. Acta Oncol 2004; 43: 316–81
- Vordermark D, Flentje M. Planning target volume definition in dose-escalation studies. Front Radiat Ther Oncol 2002; 36: 16–24
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998; 16: 139–44
- Ott C, Fulton MK. Osteoporosis risk and interest in strength training in men receiving androgen ablation therapy for locally advanced prostate cancer. J Am Acad Nurse Pract 2005; 17: 113–22
- Zelefsky MJ, Cowen D, Fuks Z, Venkatraman ES, Cowen DM, Levegrun S, et al. Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer 1999; 85: 2460–8
- Hoskin PJ. High dose rate brachytherapy boost treatment in radical radiotherapy for prostate cancer. Radiother Oncol 2000; 57: 285–8
- Boehmer D. Multi-center phase III trial on combined interstitial high-dose-rate brachytherapy plus external-beam radiotherapy versus external-beam radiotherapy alone in patients with locally advanced prostate cancer. Berlin: 2006.