Abstract
The aim of this retrospective analysis was to evaluate feasibility and effectiveness of definitive chemoradiotherapy without split-course technique in anal cancer patients. From 1993 to 2003, 81 patients were treated; 13 were excluded due to various chemotherapeutic regimes, thus 68 patients were analysed. In case of acute grade 3 toxicities, treatment was halted until improvement or resolution independent of dose. Short interruption was defined as completing treatment without exceeding eight cumulative treatment days beyond scheduled plan, other patients were considered to have had prolonged interruption. Median follow-up was 46 months. Median overall treatment time was 53 days corresponding to an interruption of eight cumulative treatment days. Thirty-five patients (51%) had treatment interruption of ≤8 days. No acute grade 4 toxicities were observed; one fatality occurred during treatment due to ileus-like symptoms according to acute grade 5 toxicity. Comparing patients with short vs. prolonged interruption 5-year actuarial rates for local control were 85% vs. 81% (p = 0.605) and for colostomy-free survival 85% vs. 87% (p = 0.762), respectively. Chemoradiotherapy with short individualised treatment interruptions seems to be feasible with acceptable acute or late toxicities. Treatment is highly effective in terms of local control and colostomy-free survival.
The treatment of cancer of the anal canal has evolved from abdominoperineal resection to definitive combined chemoradiotherapy resulting in high rate of local control while preserving a functioning anal sphincter. Nowadays surgery is only used in case of persistent or recurrent disease as salvage therapy; chemoradiotherapy with 5-Fluorouracil (5-FU) and Mitomycin C (MMC) is still considered the standard treatment for anal cancer Citation[1]. This combined treatment approach has been proven superior to radiotherapy alone in two prospective randomised trials in terms of local control Citation[2], Citation[3]. The addition of MMC to 5-FU has been found to be superior to 5-FU alone in one randomised trial Citation[4]. Due to the marked acute treatment-related toxicities a planned treatment interruption using a split-course-technique has been introduced for the prevention of severe skin reactions in the three prospective randomised trials. A break of six weeks was typically recommended between large- pelvic fields and small-volume boost fields in good responders after resolution of acute toxicities Citation[2–4]. However, there are concerns about possible adverse effects of a treatment interruption on the tumour control probability Citation[5]. The time dose factor has been suggested to play an important role for the local control in patients treated with chemoradiotherapy. In a recently published study by Graf et al. the length of the overall treatment time had a significant effect on the local control rate in patients with anal cancer Citation[6]. Deniaud-Alexandre et al. could demonstrate that when using a split-course technique the length of the gap was a significant factor on disease-free survival in patients with anal cancer Citation[7]. The goal of this retrospective study presented here was to analyse the feasibility of chemoradiotherapy with an unplanned toxicity-related individual treatment interruption in terms of acute and late toxicity and effectiveness regarding local control.
Material and methods
Between January and December 2003, 81 patients with anal cancer were treated with definitive chemoradiotherapy with two courses of 5-FU and one to two courses of MMC at our department. Thirteen patients were excluded due to treatment with chemotherapeutic regimes different from the above mentioned. Deviation from this scheme was made on an individual basis in case of contraindication or patients’ refusal. Therefore in this retrospective analysis we included 68 patients with histologically proven non-metastatic cancer of the anal canal treated with chemoradiotherapy. One fatality occurred during treatment and this patient was included in further analysis. The majority of patients (n = 37) were treated between 1996 and 1999. Clinical data were obtained retrospectively by evaluation of all patients’ records including the radiation oncologist's records on follow-up visits. Pre-treatment evaluation included patient's history, physical examination, chest radiography or computed tomography of the chest, abdominal ultrasound or computed tomography and ultrasound of the inguinal lymph nodes. All primaries were histologically proven by biopsy. The patients were analysed according to the duration of an individual treatment interruption. The patient's characteristics and clinical details are summarised in . Two patients necessitated a colostomy before start of chemoradiotherapy due to fistula formation following resection of large perianal condylomata in one patient and severe tumour-related incontinence in the other patient.
Table I. Patients’ characteristics (n = 68).
Chemotherapy
According to the chemotherapy regimens applied the patients can be separated into two treatment groups: 5-FU (1 000 mg/m2) continuous peripheral intravenous infusion for 120 hours during week 1 and 5 and MMC bolus (10 mg/m2) on day 1 (group 1, n = 37); 5-FU (1 000 mg/m2) continuous peripheral intravenous infusion for 120 hours during week 1 and 5 with MMC bolus (10 mg/m2) on day 1 and also on day 29 (group 2, n = 31). Until 1999 one course of MMC was given, a second course of MMC was given from 1999 onwards.
Radiation therapy
After CT-based treatment planning all patients received radiotherapy using 6-, 10- or 23 MV linac photons delivered by an isocentric four-field box technique using a prone (n = 59) or supine (n = 9) position. The prone position was used from 1993 to 2000 and was then switched to the supine position after changing the treatment policy to eliminate electron beams to the inguinal lymph nodes for increasing dose homogeneity. However, male patients were continuously treated in prone position to reduce the gonad dose. The treatment fields included both the primary tumour as well as the presacral and perirectal lymph nodes up to a total dose of 45 Gy. After reaching the dose of 45 Gy a boost to the primary tumour was given with reduced fields to a median total dose of 55.8 Gy (range: 32–64 Gy) depending on the T-stage (single dose 1.8 Gy). In patients with primary tumours exceeding 2 cm in maximum size or with suspected inguinal lymph node metastasis the inguinal lymph nodes were included into the photon treatment fields or were treated by electrons of appropriate energy. Lymph node metastases were suspected in case of clinically palpable inguinal lymph nodes exceeding 2 cm in size in the ultrasound or in the computed tomography. No fine needle aspiration for verification of the diagnosis was performed. In case of suspected inguinal lymph node metastasis the total dose to the inguinal lymph nodes was 55.8 Gy. For T1 and T2 tumours the upper border of the radiation field was defined by the lower edges of the ileosacral joints, for tumours infiltrating the lower rectum or in presence of iliac lymph node metastasis the fields were extended to the lower border of the fifth lumbal vertebra. The inferior border included the anal sphincter and the perineum with an appropriate safety margin.
Treatment interruption
The onset of individual treatment interruption depended on the level of acute toxicities. If the patient experienced acute grade 3 cutaneous, gastrointestinal or haematological toxicities treatment was halted until the toxicities had markedly improved or completely resolved. Treatment break was made independent of the dose actually received. The median length of treatment interruption was used as a dividing point for dichotomizing into a group with short vs. prolonged treatment interruption. Short treatment interruption was defined as completing treatment without exceeding eight cumulative treatment days beyond scheduled plan; other patients were considered to have had prolonged treatment interruption. Treatment interruption was determined by the delay of cumulative treatment days with treatment days defined as working days excluding public holidays, Saturdays and Sundays.
Follow-up and assessment
All patients being alive at the time of the data analysis were contacted by phone; additional information was obtained from the general practitioner following the patient and from the local tumour board. Median follow-up was 46 months (range: 0–126 months). At the time of the analysis 48 patients were alive; the follow-up for these patients was 57 months. In case of late toxicity of grade 2 or more an appointment was arranged and the patient was re-examined.
Statistical analysis
Statistical analysis was performed using a commercially available software package (SPSS win 11.5). All events were measured from the end of the treatment. Following end-points were analysed and compared between the different treatment groups: local control, colostomy-free survival, disease-free survival and overall survival, with death from any cause defined as event. Local failure was defined as a primary tumour persisting or recurring in the anorectal region. The actuarial rates were calculated by the product-limit method of Kaplan-Meier; differences were compared using the log-rank test. Differences between the treatment groups were tested for significance using the χ2 test for categorical factors (two to four categories) and the one-way ANOVA test for continuous variables. A p-value of less than 0.05 was considered as statistically significant.
Results
Treatment interruptions
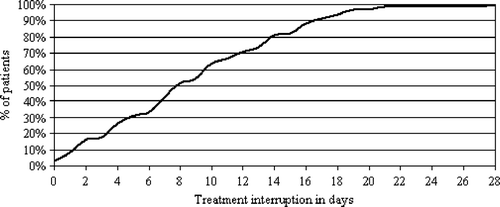
Median duration of overall treatment time was 53 days including the weekends (range: 39–78 days). Duration of individual treatment interruption was a median of eight cumulative treatment days (0–28 treatment days). The distribution of treatment interruptions is shown in . In 35 patients (51%) treatment interruption did not exceed eight cumulative treatment days. When comparing these patients with short (n = 35) vs. those with prolonged (n = 33) treatment interruption the median overall treatment time was 48 vs. 63 days corresponding to a median treatment interruption of four vs. 14 cumulative treatment days, respectively. Analyzing the cohort according to the number of MMC courses applied (1 vs. 2 courses) the median overall treatment time was 57 vs. 51 days (p = 0.061) corresponding to a median treatment interruption of nine vs. seven cumulative treatment days, respectively. No significant difference in the distribution of relevant prognostic factors (clinical T- or N-stages, radiotherapy doses) could be found between the two patient groups with short vs. prolonged treatment interruption apart from gender.
Treatment related toxicity
Regarding the treatment related toxicity acute cutaneous/subcutaneous grade 3 toxicities were seen in 19 patients and acute gastrointestinal grade 3 toxicities in 4 patients. No acute grade 4 cutaneous/subcutaneous toxicities were noted. However, one fatality of a 74-year old woman occurred during treatment. This patient had received one course of 5-FU/MMC and a cumulative dose of 32.4 Gy. At that point she presented with a grade III haematological toxicity and ileus-like symptoms that necessitated laparotomy which the patient finally succumbed after developing septic complications (acute grade 5 gastrointestinal toxicity).
If comparing patients with short vs. prolonged treatment interruption no significant difference in organ-related acute cutaneous/subcutaneous or gastrointestinal toxicities could be observed (). Analyzing the cohort according to the number of MMC courses applied (1 course (n = 37) vs. 2 courses (n = 31)) a borderline significant difference could be observed in terms of acute cutaneous (p = 0.054) and gastrointestinal toxicities (p = 0.051); we found a more pronounced acute cutaneous and gastrointestinal toxicity in patients receiving two applications of MMC.
Table II. Acute treatment-related toxicities according to length of treatment interruption (CTC version 2.0).
During the follow-up late cutaneous toxicity grade 2 was seen in six patients and late gastrointestinal toxicity grade 2 in eight patients; seven patients had a permanent colostomy; no colostomy became necessary for treatment-related toxicity. No late grade 3 or 4 cutaneous or gastrointestinal toxicities were observed (). If comparing patients with short vs. prolonged treatment interruption no significant difference in late toxicities could be observed (). If the cohort was analysed according to the number of MMC courses applied (1 vs. 2) again no statistically significant difference could be observed regarding the development of late cutaneous or gastrointestinal toxicities.
Figure 2. Perianal telangiectasia in a patient 28 months after definitive chemoradiotherapy with 55.8 Gy (grade 2 late skin reaction according to the LENT/SOMA scale)
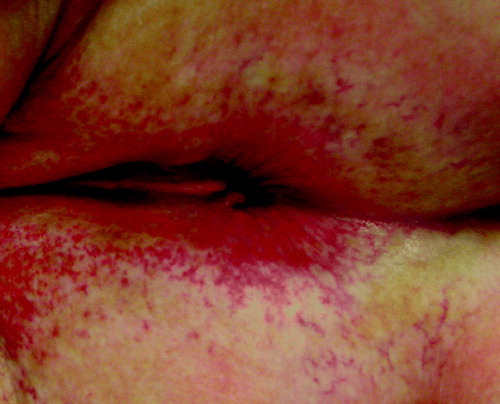
Table III. Late toxicities according to length of treatment interruption (LENT/SOMA toxicity score).
Response
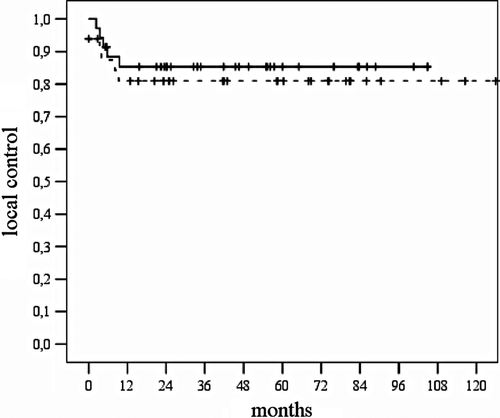
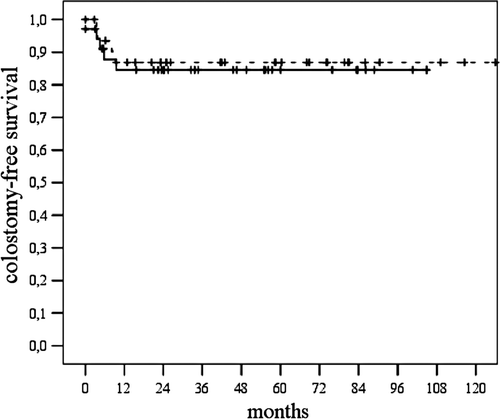
After completion of chemoradiotherapy two patients had persistent tumour and underwent consecutive abdominoperineal resection. After a median period of six months (range: 3–9 months) nine patients had local recurrent tumour, and ten patients developed distant metastases. For patients with T 1/2 tumours compared to T 3/4 tumours the corresponding 5-year actuarial rates for local control were 84% and 83% (p = 0.896), for colostomy-free survival 87% and 82% (p = 0.459), for disease specific survival 78% and 53% (p = 0.196) and for overall survival 74% and 51% (p = 0.122), respectively. If the cohort of patients was analysed separately according to the number of MMC courses applied (1 vs. 2) the corresponding 5-year actuarial rates for local control were 86% and 80% (p = 0.508), for colostomy-free survival 89% and 82% (p = 0.440), for disease specific survival 69% and 75% (p = 0.434) and for overall survival 64% and 74% (p = 0.088), respectively. Comparing patients with short vs. prolonged treatment interruption the corresponding 5-year actuarial rates for local control were 85% and 81% (p = 0.605), for colostomy-free survival 85% and 87% (p = 0.762), for disease specific survival 75% and 67% (p = 0.627) and for overall survival 71% and 63% (p = 0.593), respectively ( and ).
Discussion
Since combined chemoradiotherapy was initially introduced into clinical practice as a preoperative treatment by Nigro et al. Citation[8] the high rates of complete pathological responses found in the tumour resection specimen made this approach the treatment of choice in sphincter preserving therapy of anal cancer. Although the prognostic impact of disease-related factors such as tumour stage and tumour size are well established there is still uncertainty about optimal treatment schedule. Cummings et al. could demonstrate that chemoradiotherapy with an uninterrupted radiation course was associated with increased acute and late normal tissue morbidity. In contrast, split-course radiation and decreased fraction size resulted in decreased toxicity without worsening of tumour control probability Citation[9]. In order to improve the tumour control rate treatment strategies focused on optimising total dose, fractionation, chemotherapeutic regimen and timing of these two modalities Citation[1], Citation[10].
Additionally, the time factor has been proposed being a relevant factor for local control in patients treated with combined chemoradiotherapy. Protraction of the overall treatment time has been shown to decrease the efficacy of radiation due to repopulation of clonogenic tumour cells during treatment interruption and may lead to a decreased local control. At least for squamous cell carcinoma of the head and neck and the cervix there is evidence of the relevance of overall treatment time on the outcome. Fowler et al. could observe a significant loss of local control with prolongation of treatment time in a meta-analysis of 12 published clinical trials of head and neck cancer Citation[11]. Girinsky et al. could demonstrate an inverse correlation between treatment time and clinical outcome showing a loss of approximately 1% in local control per day for overall treatment time exceeding 52 days in patients with advanced cervical carcinomas Citation[12]. Considering the similarities of histological type and tumour biology this may be true for anal cancer as well and it is likely that shortening the overall treatment time by reducing treatment interruptions will have a similar beneficial effect on the local control rate. Graf et al. found a prolongation of 15 days in the overall treatment time to be associated with a difference in local control of 21% resulting in a loss of local control of 1.4% per day Citation[6]. However, in anal cancer the perineum, relevant parts of the inguinal nodes and vulva/vagina are included in the treatment fields causing potentially serious and severe acute cutaneous and subcutaneous toxicity which is even enhanced by the use of concurrent chemotherapy. Thus, treatment interruption due to acute toxicity is often unavoidable.
In anal cancer three prospective randomised studies used a split-course technique to allow for recovery of acute skin reactions Citation[2–4]. In these trials acute toxicity ≥grade 3 of the skin was present in 26–55% and of the gastrointestinal tract in 9–15% of the patients. In contrast, in our analysis the cumulative frequencies of acute cutaneous/subcutaneous and gastrointestinal toxicities were not increased using an individualized treatment break in comparison to published data with split-course technique. The treatment was halted at the occurrence of relevant acute grade 3 cutaneous, gastrointestinal or haematological toxicities and restarted after restoration from the toxicities with the length of cumulative treatment break determined only by the time elapsed in between. This is in contrast to other studies in which a planned treatment interruption was made independent of the level of the acute toxicities. However, comparing patients according to the number of MMC courses applied we found a more pronounced acute cutaneous and gastrointestinal toxicity in patients receiving two applications of MMC. Our results also compare favourably with the results observed in a recently published study by Vuong et al., who reported acute toxicity ≥grade 3 of the skin in 20% and of the gastrointestinal tract in 3.3% of their patients Citation[13]. In the series of Vuong et al. using conformal radiotherapy and concurrent chemotherapy all patients could complete treatment without a break.
One might argue that shortening the duration of treatment interruption may lead to increased acute side effects resulting in consequential late side effects as described in detail by Dorr et al. Citation[14]. We did not find any association between the level of acute toxicity, length of treatment interruption and level of late toxicity. The rate of late toxicities were equally distributed in the two treatment groups regarding treatment interruption, and no late cutaneous or gastrointestinal toxicity ≥grade 3 could be observed. According to the findings of Cummings et al. the risk of late toxicity ≥grade 3 in anal cancer patients may be more influenced by fraction size than by overall treatment time Citation[9].
Up to now there are only few data in anal cancer addressing the importance of the time factor on treatment outcome. Regarding the length of treatment interruption before application of the boost either by brachytherapy or external beam radiation, Peiffert et al. observed a decreased local control if the gap exceeded 63 days Citation[15] and Weber et al. Citation[16] and Deniaud-Alexandre et al. Citation[7] showed an association with improved outcome in patients treated with a gap shorter than 38 days. When considering the overall treatment time Ceresoli et al. found an inferior disease-free survival in patients with an overall treatment time exceeding 70 days Citation[17]. Allal et al. found a poorer local control rate if the overall treatment time exceeded 75 days Citation[18]. Constantinou et al. Citation[19] and Graf et al. Citation[6] observed an improved local control with an overall treatment time less than 40 days and 41 days, respectively. The 5-year local control rates observed in these series were 58–69% for patients with extended overall treatment time in contrast to 79–86% for patients with short overall treatment time Citation[6], Citation[7], Citation[15–19]. Our 5-year actuarial results for local control did not reveal any significant difference between the short vs. prolonged treatment group (85% vs. 81%). Also the number of MMC courses applied (1 vs. 2) had no influence on outcome in this analysis. These results may be attributable to the small sample size and limited number of events without sufficient power to detect a potential difference. Moreover, the overall duration of treatment interruption was comparably short in our cohort (median eight cumulative treatment days) so that a difference in treatment outcome may have been too small to be detectable in this series.
One might argue that those patients having longer unscheduled interruption probably had more chemotherapy and that more chemotherapy may compensate for a longer overall treatment time. However, this was not the case in our analysis. Evaluating the cohort according to the number of MMC courses applied (1 vs. 2 courses) the median overall treatment time was even shorter in patients receiving two courses of MMC although this was not statistically significant.
Recently published results of a prospective phase II study of the EORTC in which a planned treatment interruption was reduced to two weeks after 36 Gy could demonstrate that this treatment regime is feasible in terms of toxicities as well as local control and is now considered the new standard scheme for future clinical trials by the EORTC Citation[20]. The authors observed acute grade 3 toxicities for skin in 28% and for diarrhoea in 12% comparing well with our results with acute grade 3 toxicities for skin in 29% and for diarrhoea in 3% for patients with short treatment interruption. Bosset et al. could achieve a 3-year estimated rate for local control of 88% that was slightly better than the rate of 85% for our patients with short treatment interruption. The authors concluded that due to severity and time course of acute toxicities observed, especially the perineal skin reactions, it would be unlikely that the gap interruption may be further reduced or even omitted. This conclusion is not supported by our results showing the feasibility of chemoradiotherapy with an individualised treatment interruption.
On the other hand, improvement of local tumour control probability might be achieved by means other than shortening the overall treatment time, such as modification of chemotherapy schedule, introducing newer chemotherapeutic agents or modifying irradiation technique. For example, cisplatinum has not been available at the time of the introduction of combined chemoradiotherapy for anal cancer but has shown to be effective in squamous cell carcinoma, e.g. head and neck or cervical cancer. Some studies have evaluated the outcome of chemoradiotherapy with cisplatinum and 5-FU in anal cancer showing promising results Citation[21–23]. The role of cisplatinum in terms of effectiveness and toxicities is currently under investigation in a large randomised trial of the RTOG. In this treatment protocol a treatment break is not mandatory, while an individual treatment break of ten days is optional for severe local acute skin reactions.
In summary, we were able to demonstrate that concurrent chemoradiotherapy for anal cancer without a planned treatment interruption is feasible with acceptable acute and late toxicities. We could further show that this treatment scheme is very effective in terms of local control and colostomy-free survival. It seems reasonable to assign patients to continuous treatment with short individualised treatment interruptions if necessary as required due to acute toxicities. Considering time-dose factors this treatment scheme may lead to an improved outcome and should be investigated further.
References
- Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med 2000; 342: 792–800
- Bartelink H, Roelofson F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997; 15: 2040–9
- UKCCCR Anal Cancer Trial Working Party. Epidermoid anal cancer: Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. Lancet 1996;348:1049–54.
- Flam M, John M, Pajak TF, Petrelli N, Myerson R, Doggett S, et al. Role of Mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J Clin Oncol 1996; 14: 2527–39
- Cummings BJ, Brierley JD. Anal canal. Principles and practice of radiation oncology4th ed., CA Perez, LW Brady, EC Halperin, RK Schmidt-Ullrich. Lippincott Williams & Wilkins, Philadelphia 2004; 1630–48
- Graf R, Wust P, Hildebrandt B, Gogler H, Ullrich R, Herrmann R, et al. Impact of overall treatment time on local control of anal cancer treated with radiochemotherapy. Oncology 2003; 65: 14–22
- Deniaud-Alexandre E, Touboul E, Tiret E. Results of definitive irradiation in a series of 305 epidermoid carcinomas of the anal canal. Int J Radiat Oncol Biol Phys 2003; 56: 1259–73
- Nigro ND, Vaitkevicius VK, Considine B, Jr. Combined therapy for cancer of the anal canal: A preliminary report. Dis Colon Rectum 1974; 17: 354–6
- Cummings BJ, Keane TJ, O'Sullivan B, Wong CS, Catton CN. Epidermoid anal cancer: Treatment by radiation alone or by radiation and 5-Fluorouracil with and without Mitomycin C. Int J Radiat Oncol Biol Phys 1991; 21: 1115–25
- Esiashvili N, Landry J, Matthews RH. Carcinoma of the anus: Strategies in management. Oncologist 2002; 7: 188–99
- Fowler JF, Lindstrom MJ. Loss of local control with prolongation in radiotherapy. Int J Radiat Oncol Biol Phys 1992; 23: 457–67
- Girinsky T, Rey A, Roche B, Haie C, Gerbaulet A, Randrianarivello H, et al. Overall treatment time in advanced cervical carcinomas: A critical parameter in treatment outcome. Int J Radiat Oncol Biol Phys 1993; 27: 1051–6
- Vuong T, Devic S, Belliveau P, Muanza T, Hegyi G. Contribution of conformal therapy in the treatment of anal canal carcinoma with combined chemotherapy and radiotherapy: Results of a phase II study. Int J Radiat Oncol Biol Phys 2003; 56: 823–31
- Dorr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol 2001; 61: 223–31
- Peiffert D, Bey P, Pernot M, Guillemin F, Luporsi E, Hoffstetter S, et al. Conservative treatment by irradiation of epidermoid cancers of the anal canal: Prognostic factors of tumoral control and complications. Int J Radiat Oncol Biol Phys 1997; 37: 313–24
- Weber DC, Kurtz J, Allal AS. The impact of gap duration on local control in anal canal carcinoma treated by split-course radiotherapy and concomitant chemotherapy. Int J Radiat Oncol Biol Phys 2001; 50: 675–80
- Ceresoli GL, Ferreri AJ, Cordio S, Villa E. Role of dose intensity in conservative treatment of anal canal carcinoma. Report of 35 cases. Oncology 1998; 55: 525–32
- Allal AS, Mermillod B, Roth AD, Marti MC, Kurtz JM. The impact of treatment factors of local control in T2–T3 anal carcinomas treated by radiotherapy with or without chemotherapy. Cancer 1997; 79: 2329–35
- Constantinou EC, Daly W, Fung CY, Willett CG, Kaufman DS, DeLaney TF. Time-dose considerations in the treatment of anal cancer. Int J Radiat Oncol Biol Phys 1997; 39: 651–7
- Bosset JF, Roelofsen F, Morgan DA, Budach V, Coucke P, Jager JJ, et al. Shortened irradiation scheme, continuous infusion of 5-fluorouracil and fractionation of mitomycin C in locally advanced anal carcinomas. Results of a phase II study of the European Organization for Research and Treatment of Cancer. Radiotherapy and Gastrointestinal Cooperative Groups. Eur J Cancer 2003; 39: 45–51
- Gerard JP, Ayzac L, Hun D, Romestaing P, Coquard R, Ardiet JM, et al. Treatment of anal canal carcinoma with high dose radiation therapy and concomitant fluorouracil-cisplatinum. Long-term results in 95 patients. Radiother Oncol 1998; 46: 249–56
- Hung A, Crane C, Delclos M, Ballo M, Ajani J, Lin E, et al. Cisplatin-based combined modality therapy for anal carcinoma: A wider therapeutic index. Cancer 2003; 97: 1195–202
- Peiffert D, Giovannini M, Ducreux M, Michel P, Francois E, Lemanski C, et al. Digestive Tumours Group of the French ‘Federation Nationale des Centres de Lutte Contre le Cancer’. High-dose radiation therapy and neoadjuvant plus concomitant chemotherapy with 5-fluorouracil and cisplatin in patients with locally advanced squamous-cell anal canal cancer: Final results of a phase II study. Ann Oncol 2001; 12: 397–404