Abstract
Endocrine ophthalmopathy is to some degree present in most patients with Graves’ disease. In few cases, a severe form of the condition develops and in the majority of these cases, the course of the eye problems has been influenced by the treatment for thyrotoxicosis. In this regard, radioiodine therapy has been increasingly recognized as carrying a special risk. Here, the current understanding of endocrine ophthalmopathy and the risks associated with the development of severe eye disease are discussed. The results of a retrospective investigation of patients with severe eye disease in our hospital, and the experience with corticosteroid administration following radioiodine in order to reduce the risk of ophthalmopathy, are also presented.
Radioiodine has been used for treatment of thyrotoxicosis due to Graves’ disease and toxic nodular goiter since more than 50 years Citation[1], Citation[2]. The possibility of a special influence of radioiodine on the course of endocrine ophthalmopathy (EO), the unique component of Graves’ disease, remained under debate for many years, although an insight had grown (exemplified in Citation[3–5]) that this treatment modality carried an extra risk compared with thyrostatic drugs or thyroid surgery. In the 1980 – 1990's randomized controlled trials Citation[6–8] added the conclusive evidence. Today the risk of radioiodine is recognized, but the mechanisms by which it can promote EO are not fully understood.
Development of current knowledge of EO and radioiodine therapy
The reason why it took so long to establish a relationship between treatment for thyrotoxicosis and course of EO most likely reflect the heterogeneous nature of the disease, the different susceptibility for EO among patients, and the role of exogenous factors, e.g. smoking Citation[9], Citation[10] and negative stress Citation[11]. Furthermore, the eye signs were wrongly claimed to follow a seemingly unpredictable course, and to be evenly present prior to, at diagnosis, and after onset of thyrotoxicosis. Today, such statements are no longer valid as a result of reports on well characterized patients (including data on thyroid hormone balance, TSH receptor antibodies) with careful descriptions of the history of treatment of the underlying thyroid disease.
The development of precise assays of thyroid hormones and, in particular sensitive tests of thyrotropin, were important early steps forward for detailed characterizations of patients. The identification of TSH receptor antibodies, first as “long acting thyroid stimulator” activity in serum, and subsequently as antibodies binding to TSH receptors on thyrocyte plasma membranes, and measurable by radioreceptor Citation[12] and biological assays, likewise have been important advances. In the 1980's commercial assays became available and today there are several user-friendly radioimmunoassay or elisas on the market for measurements of the TSH receptor antibodies. Other major advances for the understanding of Graves’ disease and EO was the cloning of the TSH receptor Citation[13], Citation[14] and in recent years, the identification of TSH receptor expression in extra-thyroidal tissue, notably retro-orbital fibroblasts and fat cells Citation[15–17].
Key steps in development of current knowledge regarding EO and radioiodine therapy
Radioimmunoassays for precise measurements of thyroid hormones
Sensitive assays for measurements of suppressed thyrotropin concentrations
Discovery of TSH receptor antibodies and the production of research assays
Commercial TRAb assays
Recognition of the important role of TSH receptor activation and thyroid disease activity for development of EO
Establishing radioiodine as a risk for promoting eye disease in randomized trials
Cloning of the TSH receptor
Expression of TSH receptors in non-thyroidal tissue, e.g. retroorbital cells
Endocrine ophthalmopathy
Most patients with Graves’ disease present with some ocular involvement, endocrine opthalmopathy, which is due to an inflammation of the eye muscle(s), retroorbital fat and connective tissue. There is irritation of the eyes with teariness, some eye lid retraction, and variable degrees of eye lid edema and periorbital swelling. The affected eye muscles become swollen and their motility impaired such that diplopia may occur. The enlargement of the muscles and retroorbital tissues leads to proptosis. In severe EO, the retroorbital swelling may cause compression of the optic nerve with impairment of the visual field and/or visual acuity. The basis of the eye involvement remains still not well understood. Microscopically, some inflammatory cells, lymphocytes, granulocytes as well as macrophages are found in the edematous eye muscles and orbital fat. The thinking has long been that the eye muscle and fat tissue contain some thyroid-related structures. The findings of TSH receptor expression in retro orbital tissues have given strong support to such a notion.
The fact that children with neonatal thyrotoxicosis caused by intrauterine transfer of maternal TSH receptor antibodies develop exophthalmos illustrates that fetal orbital tissues respond to TSH receptor activation in the absence of autologous Graves’ disease. Exophthalmos is feature also in newborns with congential thyrotoxicosis due to an activating mutation in the TSH receptor gene Citation[18], Citation[19]. The description of exophthalmos in a child with high TSH levels due to thyroid hormone resistance Citation[20] suggests that also in this situation stimulation of fetal retro-orbital TSH receptors produces exophthalmos.
EO in hypothyroidism
Endocrine ophthalmopathy (exophthalmos and inflammation) may in rare cases develop in hypothyroid patients without a history of thyrotoxicosis. In early literature such cases were thought to suffer from Hashimoto's thyroiditis Citation[21], Citation[22]. When examined for long acting thyroid stimulator, however, positive titers were found. Today, the rare cases presenting with hypothyroidism and concomitant endocrine ophthalmopathy without a period of prior thyrotoxicosis, without exception display marked titers of TSH receptor antibodies. These patients can be regarded as having Graves’ disease and concomitant autoimmune thyroiditis. Some of them may later develop thyrotoxicosis, due to of a shift in the antibody profile from blocking to stimulatory, or rather due to remission of the thyroiditis component.
TSH receptor antibodies and radioiodine treatment
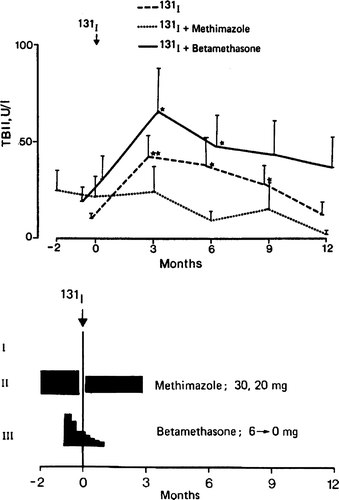
The TSH receptor antibody population in Graves’ disease often contains both stimulatory and blocking antibodies, with a predominance of the stimulatory ones. Changes in the antibody spectrum have been observed following radioiodine treatment with blocking antibodies appearing, in some cases contributing to a rapid development of hypothyroidism after treatment Citation[23], Citation[24]. The typical rise in antibody titers following radioiodine most likely represent a boost of B-lymphocytes by antigen released from radiation-damaged thyrocytes. Interestingly, the rise in TSH receptor antibodies can be prevented by giving methimazole before and after radioiodine as illustrated in Citation[25]. In this study of 60 patients, methimazole was given for two months prior to radioiodine, discontinued for a week before the dose of radioiodine and then given for another three months starting on the day after radioiodine. Surprisingly, betametasone treatment given over six weeks in conjunction with the radioiodine dose increased rather than decreased the autoantibody titer in spite of a significant and longstanding reduction of circulating total IgG Citation[25].
Figure 1. Effects of radioiodine treatment (350 MBq 131I) of TSH receptor antibody levels (TSH binding inhibition immunoglobulin, TBII) in patients with newly diagnosed Graves disease randomized to three treatment groups, I radioiodine only (n = 23), II methimazole and radioiodine (n = 17), III betamethasone (n = 20). From reference 25 with permission from J Clin Endocrinol Metab.
Radioiodine-induced conversion of TSH receptor antibody-negative patients
TSH receptor antibodies, as measured by the tests available today, are present at diagnosis in the vast majority of patients with Graves’ disease. There are occasional patients who present with a history of thyrotoxicosis of short duration, heredity for Graves’ disease and mild eye signs including eye lid retraction typical of Graves’ disease, but no detectable serum TSH receptor antibodies. Such patients will develop antibody titers if given radioiodine. This was described by us in paper from 1994 Citation[26], in which we reported nine cases with TSH receptor antibody-negative clinical Graves’ disease. Eight of the patients had bilateral diffuse thyroid uptake at scintigraphy and one had a uninodular uptake but similar to the other eight, a history, heredity and clinical signs suggestive of Graves’ disease. Presumably, this patient had two disorders, nodular goiter and Graves’ disease, with the nodule being highly sensitive to a low (undetectable) TSH receptor antibody titer. It has been suggested that radioiodine could precipitate de novo Graves’ disease, but this seems questionable as there is a vast clinical experience and also a number of studies of radioiodine treatment of nodular goiter without any observations of induction of Graves’ disease. Einhorn et al. Citation[27] showed in an early study that radioiodine treatment of toxic nodular goiter gave rise to no or minor titers of antibodies against thyroglobulin and/or thyroid microsomes but not to Graves’ disease.
Importance of TSH receptor activation and thyroid tissue activation in the development of EO
Patients with Graves’ disease have variable degrees of eye signs at diagnosis, which in most cases remit as the thyrotoxicosis is cured. In few patients, however, severe eye disease with marked periorbital swelling, proptosis, pain and in some cases also optic nerve damage develops after the start of treatment for thyrotoxicosis.
It was long thought that the treatment modality had no influence on the course of EO. In a retrospective evaluation of 30 consecutive cases with the most severe endocrine ophthalmopathy () treated at our hospital in the 1980's an interesting picture emerged Citation[28]. In most cases a treatment-induced period of hypothyroidism was identified regardless of the mode of treatment (antithyroid drugs, radioiodine or thyroid surgery). Severe EO had occurred in another eight patients treated with radioiodine although there was no documented hypothyroid episode. In three more cases, treatment of thyrotoxicosis with antithyroid drugs had been stopped because of drug reactions, leading to recurrent thyrotoxicosis which was followed by the development of severe EO (). Thus, the records revealed that eye signs often had deteriorated after episodes of marked changes in thyroid function, in particular treatment-induced hypothyroid episodes, or episodes in which the thyroid hormone values had changed from elevated to normal and back to elevated in a short period of time, i.e. rapid shifts in thyroid hormone status seemed to somehow have a negative influence.
Table I. Thirty consecutive patients with severe endocrine ophthalmopathy.
Table II. Treatment-induced thyroid activation in 30 patients with severe endocrine ophthalmopathy.
It appeared that the common denominator among these cases of severe EO was an activation of the thyroid (a rise in TSH receptor antibodies, in hypothyroid cases also TSH), implying TSH receptor activation as a factor of great importance for the evolvement of severe eye disease Citation[28]. In support of this concept, the value of early thyroxine administration after radioiodine treatment has been pointed out by Tallstedt et al. Citation[29] and the link between TSH receptor antibody levels and eye signs recently corroborated by Eckstein et al. Citation[30] who investigated the severity of clinical eye disease in 108 patients with Graves’ disease after treatment with steroids. They noted an association between TSH receptor antibody prevalence and activity/severity of EO in patients independently of antithyroid drug treatment, thyroidectomy or radioiodine therapy.
In 1989 we emphasized in a Letter to the Lancet the importance of establishing ‘thyroid rest’ for the course of EO: “We therefore prescribe thyrostatic drugs, combined with thyroxine, to all patients with TSH receptor antibodies, including those who are euthyroid after previous thyroid surgery or radioiodine therapy, to prevent stimulation of the thyroid” Citation[5]. Nowadays, this view is shared by an increasing number of colleagues and not unlikely a factor contributing to the encouraging trend with a fall in the number of patients with severe eye disease seen at our hospital and other referral centers in Sweden.
Corticosteroid administration after radioiodine treatment
If radioiodine is given to a patient with some eye signs, a course of glucocorticoids is of great value to lower the risk of radioiodine-induced aggravation of eye disease Citation[6]. At our hospital we have since 1990 used a protocol () that differs somewhat from that described by Bartalena et al. in 1989 Citation[6]. We limit the use of radioiodine (Rx) to select cases with mild and moderate EO and do not use it in patients with marked eye signs. With this strategy we have not encountered problems of severe eye disease developing, although some patients with “active” EO (eyelid oedema, conjunctivitis, tears and/or pain) experience transient worsening of eye symptoms even though they receive the steroid regimen. At the Department of Endocrinology, Uppsala University Hospital, 27 patients were treated between 1990 and year 2000 according to the prednisolone protocol. At the time of Rx, the patients had been treated with thyrostatic drugs for a median of ten months. The TSH receptor antibodies (TRAK) were positive in 25 of 27 patients, and the median total T3 and fT4 values were 2.7 nmol/L and 27 pmol/L, respectively. Seventeen of the patients had active and ten inactive EO, and at a follow-up after 6 – 12 months 5 and 1, respectively, had experienced a transient worsening of the eye symptoms. The “worsening” group had TSH receptor antibodies of 50 (median) at Rx and the “non-worsening” group 20 (ref < 9 U/L).
Table III. Protocols for glucocorticoid-treatment of patients with eye signs subjected to radioiodine therapy.
Concluding comments
Today, we know that severe endocrine ophthalmopathy is a condition that almost without exception only develops after the start of treatment for thyrotoxicosis, and that radioiodine therapy is the treatment modality that carries the greatest risk. Furthermore, we know that it is important to render a hyperthyroid patient stably euthyroid without episodes of treatment-induced hypothyroidism. It is also important not to give a patient with eye signs very high doses of thyrostatic drugs as adverse reactions, if they occur, often lead to fluctuating thyroid hormone levels and thereby increase the risk of subsequent eye complications. In patients with eye signs present already at diagnosis of thyrotoxicosis, moderate doses of prednisolone, 15 – 20 mg tapered over three months, could be used as a supplement to thyrostatic drugs early in the course to reduce the risk of developing more severe eye problems. Likewise, prednisolone is of use to lower the risk of ophthalmopathy after radioiodine treatment. Finally, in cases with severe eye signs and a thyrotoxicosis that do not respond well to thyrostatic drugs, we refer the patient to near total/total thyroidectomy Citation[31], Citation[32].
References
- Chapman EM. History of the discovery and early use of radioactive iodine. JAMA 1983; 250: 2042–4
- Sawin CT, Becker DV. Radioiodine and the treatment of hyperthyroidism: The early history. Thyroid 1997; 7: 163–76
- Pequegnat EP, Mayberry WE, McConahey WM, Wyse EP. Large doses of radioiodide in Graves’ disease: Effect on ophthalmopathy and long-acting thyroid stimulator. Mayo Clin Proc 1967; 42: 802–11
- Vestergaard H, Laurberg P. Radioiodine and aggravation of Graves’ ophthalmopathy. Lancet 1989; 2: 47
- Karlsson FA, Westermark K, Dahlberg PA, Jansson R, Enoksson P. Ophthalmopathy and thyroid stimulation. Lancet 1989; 2: 691
- Bartalena L, Marcocci C, Bogazzi F, Panicucci M, Lepri A, Pinchera A. Use of corticosteroids to prevent progression of Graves’ ophthalmopathy after radioiodine therapy for hyperthyroidism. N Engl J Med 1989; 321: 1349–52
- Tallstedt L, Lundell G, Torring O, Wallin G, Ljunggren JG, Blomgren H, et al. Occurrence of ophthalmopathy after treatment for Graves’ hyperthyroidism. The Thyroid Study Group. N Engl J Med 1992; 326: 1733–8
- Bartalena L, Marcocci C, Bogazzi F, Manetti L, Tanda ML, Dell'Unto E, et al. Relation between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med 1998; 338: 73–8
- Hagg E, Asplund K. Is endocrine ophthalmopathy related to smoking?. Br Med J 1987; 295: 634–5
- Vestergaard P. Smoking and thyroid disorders- a meta-analysis. Eur J Endocrinol 2002; 146: 153–61
- Winsa B, Adami HO, Bergstrom R, Gamstedt A, Dahlberg PA, Adamson U, et al. Stressful life events and Graves’ disease. Lancet 1991; 338: 1475–9
- Smith BR, Hall R. Binding of thyroid stimulators to thyroid membranes. FEBS Lett 1974; 42: 301–4
- Parmentier M, Libert F, Maenhaut C, Lefort A, Gerard C, Perret J, et al. Molecular cloning of the thyrotropin receptor. Science 1989; 246: 1620–2
- Nagayama Y, Kaufman KD, Seto P, Rapoport B. Molecular cloning, sequence and functional expression of the cDNA for the human thyrotropin receptor. Biochem Biophys Res Commun 1989; 165: 1184–90
- Feliciello A, Porcellini A, Ciullo I, Bonavolonta G, Avvedimento EV, Fenzi G. Expression of thyrotropin-receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet 1993; 342: 337–8
- Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves’ orbital adipose/connective tissues: Potential autoantigen in Graves’ ophthalmopathy. J Clin Endocrinol Metab 1998; 83: 998–1002
- Starkey KJ, Janezic A, Jones G, Jordan N, Baker G, Ludgate M. Adipose thyrotrophin receptor expression is elevated in Graves’ and thyroid eye diseases ex vivo and indicates adipogenesis in progress in vivo. J Mol Endocrinol 2003; 30: 369–80
- Kopp P, van Sande J, Parma J, Duprez L, Gerber H, Joss E, et al. Brief report: Congenital hyperthyroidism caused by a mutation in the thyrotropin-receptor gene. N Engl J Med 1995; 332: 150–4
- de Roux N, Polak M, Couet J, Leger J, Czernichow P, Milgrom E, et al. A neomutation of the thyroid-stimulating hormone receptor in a severe neonatal hyperthyroidism. J Clin Endocrinol Metab 1996; 81: 2023–6
- Ono S, Schwartz ID, Mueller OT, Root AW, Usala SJ, Bercu BB. Homozygosity for a dominant negative thyroid hormone receptor gene responsible for generalized resistance to thyroid hormone. J Clin Endocrinol Metab 1991; 73: 990–4
- Wyse EP, McConahey WM, Woolner LB, Scholz DA, Kearns TP. Ophthalmopathy without hyperthyroidism in patients with histologic Hashimoto's thyroiditis. J Clin Endocrinol Metab 1968; 28: 1623–9
- Sung LC, McDougall IR. Graves’ hyperthyroidism. Spontaneous occurrence after autoimmune hypothyroidism with persistent infiltrative ophthalmopathy. Arch Intern Med 1978; 138: 1009–10
- Bech K, Bliddal H, Siersbaek-Nielsen K, Friis T. Production of non-stimulatory immunoglobulins that inhibit TSH binding in Graves’ disease after radioiodine administration. Clin Endocrinol (Oxf) 1982; 17: 395–402
- Yoshida K, Aizawa Y, Kaise N, Fukazawa H, Kiso Y, Sayama N, et al. Role of thyroid-stimulating blocking antibody in patients who developed hypothyroidism within one year after 131I treatment for Graves’ disease. Clin Endocrinol (Oxf) 1998; 48: 17–22
- Gamstedt A, Wadman B, Karlsson A. Methimazole, but not betamethasone, prevents 131I treatment-induced rises in thyrotropin receptor autoantibodies in hyperthyroid Graves’ disease. J Clin Endocrinol Metab 1986; 62: 773–7
- Ilicki A, Gamstedt A, Karlsson FA. Hyperthyroid Graves’ disease without detectable thyrotropin receptor antibodies. J Clin Endocrinol Metab 1992; 74: 1090–4
- Einhorn J, Fagraeus A, Jonsson J. Thyroid antibodies after 131-I treatment for hyperthyroidism. J Clin Endocrinol Metab 1965; 25: 1218–24
- Karlsson FA, Dahlberg PA, Jansson R, Westermark K, Enoksson P. Importance of TSH receptor activation in the development of severe endocrine ophthalmopathy. Acta Endocrinol (Copenh) 1989; 121(Suppl 2)132–41
- Tallstedt L, Lundell G, Blomgren H, Bring J. Does early administration of thyroxine reduce the development of Graves’ ophthalmopathy after radioiodine treatment?. Eur J Endocrinol 1994; 130: 494–7
- Eckstein AK, Plicht M, Lax H, Hirche H, Quadbeck B, Mann K, et al. Clinical results of anti-inflammatory therapy in Graves’ ophthalmopathy and association with thyroidal autoantibodies. Clin Endocrinol (Oxf) 2004; 61: 612–8
- Winsa B, Rastad J, Akerstrom G, Johansson H, Westermark K, Karlsson FA. Retrospective evaluation of subtotal and total thyroidectomy in Graves’ disease with and without endocrine ophthalmopathy. Eur J Endocrinol 1995; 132: 406–12
- Jarhult J, Rudberg C, Larsson E, Selvander H, Sjovall K, Winsa B, et al. Graves’ disease with moderate-severe endocrine ophthalmopathy-long term results of a prospective, randomized study of total or subtotal thyroid resection. Thyroid. 2005; 15: 1157–64