Abstract
Cancers of major and minor salivary glands represent a histopathologic challenge in two major respects. The first challenge is the complexity of morphologic features and overlapping of histologic patterns in the different tumor entities many of which are relatively rare. The number of separate tumor entities to be considered in differential diagnosis has greatly increased in the two latest WHO classification systems CitationCitation (Table I). The second challenge is prognostication based on histopathology. The clinical experience is that behavior of some salivary gland carcinomas does not correlate well with their histopathologic classification, and that tumors classified within the same category may exhibit quite different clinical outcomes. However, recent advances in histopathological classification have been combined with new tools in immunohistochemical diagnosis and prognostication including cell-proliferation markers, myoepithelial antigens, matrix metalloproteinases, steroid receptors, growth factors and their receptors. These have improved our possibilities for more specific choices in the treatment of a variety of salivary gland carcinomas. This paper will give an overview on recent developments in histopathological classification, prognostication, and molecular pathology of salivary gland cancer.
Histopathological classification of salivary gland carcinoma
The basic structural component of salivary glands is the acinar-ductal unit that comprises acinar cells on the inner aspect of the acini, while ductal epithelial cells line the inner aspect of striated ducts and excretory ducts. Myoepithelial cells surround the outer aspects of acini and are found scattered on the outer aspect of intercalated and striated ducts. Basal (reserve) cells line the outer aspect of excretory ducts. The above cell types form the basis of salivary gland carcinomas; the neoplastic process can arise from acinar/ductal or myoepithelial cells alone, or, much more commonly both the inner and outer cell types participate in the tumors.
The recent WHO Classification of Head and Neck Tumors (2005) contains 24 different entities of salivary gland carcinoma (). However, a simplified but practical grouping of salivary gland carcinomas may be based on the degree of malignancy. It comprises high-grade and low-grade carcinomas, as well as a group of tumors with intermediate/interdeterminate malignancy. The most important high-grade carcinomas are mucoepidermoid carcinoma (MEC)-high grade, salivary duct carcinoma (SDC), and carcinoma-ex-pleomorphic adenoma. The most important low-grade carcinomas include MEC-low grade, acinic cell carcinoma (AcCC) and polymorphous low-grade adenocarcinoma (PLGA). The third category comprises especially adenoid cystic carcinoma (AdCC) with a long-term poor prognosis, as well as myoepithelial carcinoma and epithelial-myoepithelial carcinoma where the spectrum of behavior is not well known yet.
Table I. WHO histological classification of tumors of the salivary glands
Another practical grouping of salivary gland carcinomas is based on the relative frequency of their occurrence. In a recent whole population-based study of all epithelial salivary gland carcinomas in Finland during 1991–1996 (237 cases), we distinguished AdCC (27.0% of all SGC), MEC (19.2%), and AcCC (17.0%) as a group of most frequently occurring carcinomas Citation[3]. The next group of less frequent but not unusual carcinomas comprised SDC (5.4%), PLGA (3.3%) and carcinoma-ex-pleomorphic adenoma (3.3%). All other types of salivary gland carcinoma occurred clearly less frequently, and many of them were quite rare.
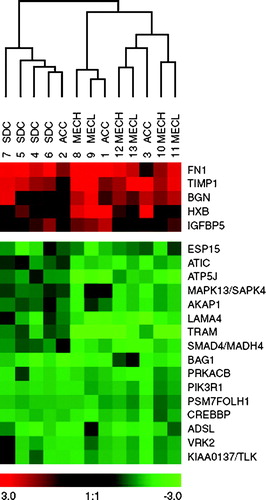
Figure 1. Gene expression profiles relating to acinic cell carcinoma (ACC), salivary duct carcinoma (SDC), low-grade mucoepidermoid carcinoma (MECL) and high-grade mucoepidermoid carcinoma (MECH) obtained by means of cDNA arrays and visualized using Treeview. Dendrogram of hierarchical clustering of gene expression showing relationships between 13 carcinoma cases. Carcinoma samples in columns (with patient code numbers), and gene abbreviations in rows. Gene expression is coded from overexpressed to underexpressed (bar; red to green) with color intensity correlating with degree of gene expression. Five genes are overexpressed and 16 genes underexpressed in all carcinoma types. Reprinted Cancer and Cytogenetics 156: 104_113, 2005.
Mucoepidermoid carcinoma (MEC)
MEC contains squamous cells, mucus-producing cells and cells of intermediate type. Clear cell change is often seen in squamous and intermediate cells, and MEC may take the form of a clear cell carcinoma. Oncocytic metaplasia is also frequent. Keratinization is extremely rare. MEC may be cystic, and tends then to have more numerous mucous cells.
Thus, MEC has many possible histopathologic patterns emphasizing the need for immunohistochemical markers for its microscopic identification. However, no specific or reliable markers have been available. Mucin stains mucicarmine, AB-PAS or MUC-antigens are helpful particularly if the mucous cells are few Citation[4], Citation[5]. A peripheral orientation of cytokeratin 14 in tumor cell islands has been described Citation[6], but not always seen. Recent progress in molecular cytogenetic studies indicates that low-grade MEC express a MECT1-MAML2 fusion oncogene derived from the translocation t(11;19)(q21;p13) [7,8,9; for details see p. 5]. Interestingly, the fusion is seen in low-grade MEC only, and antibodies to the fusion protein are promising for specific identification of this form of salivary gland cancer.
In recent decades, a number of grading systems have been devised to predict the behavior of MEC. Grading has been based on extent of the cystic component, neural invasion, necrosis, mitotic activity and cytological pleomorphism Citation[10], Citation[11]. To increase the degree of repeatability in grading, pattern of tumor infiltration, vascular and bony invasion were added recently Citation[12]. A simple cell cycle-based Ki-67 (MIB-1 antibody) proliferation index is also valuable in identifying high-grade MEC with increased recurrence, metastasis and decreased patient survival Citation[13].
For characterization of mucoepidermoid carcinomas, immunohistochemistry for mucins MUC1, MUC2, MUC4, and MUC5AC was studied recently Citation[5]. MUC4 staining was related to tumor differentiation, while staining for MUC1 indicated a worse prognosis. Staining for MUC5AC appeared helpful in differential diagnosis of high-grade MEC from squamous cell carcinoma.
Acinic cell carcinoma
Acinic cell carcinoma (AcCC) is the only salivary gland carcinoma that demonstrates serous acinar cell differentiation. AcCC may assume different growth patterns including solid, microcystic, papillary-cystic and follicular, and may contain a number of different cell type – acinar, non-specific glandular, intercalated ductal, microvesicular, hobnail or clear cells. This multitude of morphologies calls for diagnostic markers, but immunohistochemistry has been of limited value so far. Stainings for amylase, CEA or BMP-6 Citation[14] have been suggested. Importantly, the multitude of morphologic variations in AcCC do not correlate with the clinical outcome. Two separate studies on cell cycle antigens, however, have indicated that Ki-67 is an independent prognosticator in AcCC Citation[15], Citation[16]. Cases with a proliferation index < 5% were cured by complete local excision, while more than half of tumors with indices above this threshold recurred and/or metastasized.
Salivary duct carcinoma
Salivary duct carcinoma (SDC) arises almost always in the parotid gland with at least 3:1 male preponderance. It can also develop in pleomorphic adenomas. The histopathologic appearance of SDC shows a striking resemblance to ductal carcinoma of the breast. Recently, it was revealed that many histopathologic growth patterns of the ductal carcinoma of breast are also recapitulated in SDC, including intraductal-like (DCIS-like), micropapillary, mucin-rich and sarcomatoid patterns Citation[2], Citation[17–20]. In immunohistochemistry, this salivary malignancy shows frequent (nearly 100%) positivity for androgen receptors (AR), even in female patients Citation[21], while estrogen and progesteron receptors are negative. SDC are usually distinctly positive for HER-2/neu protein but the gene amplification has been reported in less than half of them Citation[22]. SDC is a high-grade carcinoma with a poor prognosis. Recent cases Citation[23] of low-grade SDC could be better classified as low-grade cribriform cystadenocarcinoma. At present, radical surgery combined with radiotherapy is the preferred treatment of SDC.
Myoepithelial carcinoma
In the WHO 2005 classification, myoepithelial carcinoma represents a malignancy with only (or predominantly) myoepithelial neoplastic cells Citation[2]. Previous designations included malignant myoepithelioma (and clear cell malignant myoepithelioma) Citation[1]. Myoepithelial carcinomas occur in parotid, submandibular and minor glands. Apparently, a large proportion of these tumors arise in pre-existing pleomorphic adenomas or myoepitheliomas Citation[24–27]. The histomorphology is characteristically nodular or multinodular with invasive growth. Myoepithelial carcinomas have varied cell types from spindled to epithelioid, plasmacytoid and stellate forms as well as cells with clear cytoplasm. Nuclear morphology can also be variable from almost uniform to highly pleomorphic. There is usually tumor-associated extracellular matrix, of myxoid or hyalinized collagenous type. In immunohistochemistry, positivity for myoepithelial markers including calponin, smooth muscle actin, cytokeratin 14, and p63 is often patchy and in a given tumor may only express a few of them. Significant proportions of myoepithelial carcinomas show some positivity for p63 Citation[28]. Ki-67 index may be high, and indices above 10% should raise concern of malignancy Citation[29]. The prognosis of myoepithelial carcinoma is variable – according to AFIP Citation[11] one third of patients die of disease, one third show local recurrences while one third have no signs of disease in follow-up. However, prognostic markers for myoepithelial carcinoma remain uncertain as histologic grade, tumor size, perineural and vascular invasion did not predict clinical outcome Citation[27]. Treatment is surgical, and the value of other modalities is not established.
Polymorphous low-grade adenocarcinoma
Polymorphous low-grade adenocarcinoma arises almost exclusively in intraoral minor salivary glands, particularly in the palate. The histopathology of this neoplasia is characterized by parameters of its name, namely morphological diversity, cytological uniformity and invasive growth Citation[2]. Growth patterns are numerous, and some of them such as Indian filing of cells with bland morphology and concentric perineural growth bear resemblance to lobular carcinoma of the breast. Mitoses are rare and Ki-67 indices are very low Citation[30]. PLGA is a low-grade malignancy with tendency for local recurrences and sometimes locoregional metastases, but distant metastases are unusual. Recurrences seem to be usually due to incomplete primary excision Citation[31], Citation[32].
The differential diagnosis of a palatal salivary gland tumor comprises adenoid cystic carcinoma (AdCC), pleomorphic adenoma and PLGA, and may sometimes be problematic due to the usually small size of these biopsies. Ki-67 index can usually be helpful in differentiating between AdCC and PLGA Citation[30]. Lack of myxochondroid matrix may help to differentiate between PLGA and pleomorphic adenoma. Lack of a peritumoral capsule in intraoral pleomorphic adenomas may complicate the evaluation of possible invasive growth in a biopsy. PLGA with extensive papillary growth pattern behave somewhat more aggressively, and may better be classified as papillary cystadenocarcinomas Citation[2].
Cribriform adenocarcinoma of the tongue
The WHO 2005 classification introduces cribriform adenocarcinoma of the tongue (CAT), a newly described malignancy that shares some histological features with PLGA. CAT was described in base of the tongue, and it usually shows metastases in neck lymph nodes at the time of presentation. No distant spread was reported and all patients did well in follow-up without recurrences or new metastases Citation[33].
Growth patterns are microcystic and/or cribriform/tubular. In immunohistochemistry, cytokeratins and S-100 are strongly positive, while myoepithelial markers show only limited patchy positivity. Treatment is surgical excision and radiotherapy.
Clear cell carcinoma, NOS (Hyalinizing clear cell carcinoma)
This distinct clear cell carcinoma has a purely epithelial phenotype, and was named as hyalinizing clear cell carcinoma Citation[34]. The entity was introduced in WHO 2005 classification under a synonymous term clear cell carcinoma, not otherwise specified (). The tumor usually arises in minor salivary glands. Histopathologically, groups of clear cells are divided into compartments by collagenous septa. In immunohistochemistry, the clear cells are stained with various cytokeratins and EMA but they are consistently negative for myoepithelial markers Citation[34]. Behavior is of low-grade malignancy. Treatment is surgical removal.
Challenge of molecular pathology in salivary gland carcinoma
Despite advances in protein markers, genetic events underlying the development and progression of salivary gland cancer are largely unknown. For defining new prognostic and diagnostic markers and designing targeted therapeutic interventions, there is a clear need for better understanding of such events.
Alterations in gene expression in salivary gland carcinoma
Abnormal gene expression in adenoid cystic carcinoma (AdCC) has been studied using oligonucleotide arrays Citation[35]. The most overexpressed genes coded for basement membrane and extracellular matrix proteins of myoepithelial differentiation, such as laminin-β1, versican, biglycan and type IV collagen-α1. Other overexpressed genes included transcription factors SOX-4 and AP-2 family, and members of the Wnt/beta-catenin signaling pathway such as casein kinase 1, epsilon and frizzled-7. The most underexpressed genes included genes encoding for proteins of acinar-type differentiation such as amylase, carbonic anhydrase and salivary proline-rich proteins. In AdCC, loss of heterozygosity frequently occurs in chromosome 6q23-25, correlating with prognostic parameters Citation[36].
Major salivary gland carcinomas with epithelial differentiation (acinar or ductal type) include mucoepidermoid carcinoma (MEC), acinic cell carcinoma (AcCC) and salivary duct carcinoma (SDC). Gene expression profiles have been studied in all three and compared. Only five genes were jointly overexpressed including fibronectin (FN1), (; Citation[37])tissue metalloproteinase inhibitor-1 (TIMP1), biglycan (BGN), tenascin C (HXB), and insulin-like growth-factor binding protein-5 (IGFBP5). Sixteen genes, i.e. KIAA0137/TLK1, VRK2, ADSL, CREBBP, PSM/FOLH, PIK3R1, PRKACB, BAG1, SMAD4/MADH4, TRAM, LAMA4, AKAP1, MAPK13, ATP5J, ATIC, and EPS15, were jointly underexpressed.
In hierarchical clustering, low-grade and high-grade MEC clustered together separately from closely clustered SDC Citation[37]. Clustering of SDC and AcCC, on the other hand, also showed each entity clustering together and separately from the other. Genes differently expressed between these carcinomas were identified by significance analysis of microarrays (SAM). In SAM, five genes, MMP11, DAP12, KIAA0324, FASN and CASP10, were overexpressed by SDC. Eight genes, including IL-6 and KRT14 were overexpressed by MEC. Another 14 genes that are mainly involved in DNA modification, such as NME4, NTHL1, RBBP4, HMG17 and NDP52, were underexpressed by MEC. Quantitative RT-PCR and immunohistochemistry were used to confirm the results concerning e.g. overexpression of FN1 and TIMP1, underexpression of PSM/FOLH1 and MADH4/SMAD4, as well as the differences of expression profiles of IL-6, CASP10 and KRT14 between SDC and MEC Citation[37].
Major differences between predominantly myoepithelial and predominantly epithelial salivary gland carcinomas also exist. In MEC and AdCC, the expression of major effector molecules such as erbB-2, erbB-3, epidermal growth factor receptor and transforming growth factor-α is quite dissimilar Citation[38].
Genomic copy number, translocations and epigenetic mechanisms
Recent studies on adenoid cystic carcinoma have characterized previously identified deletions at 12q13.11-q13.13 and 12q24.32-q24.33. Expression arrays showed that several genes in these areas have a significantly down-regulated transcription suggesting them as candidates for further studies Citation[39]. Such genes at 12q12-q13 include e.g. homeobox C5 (HOXC5) and activin A receptor (ACVRL1), and at 12q24 transcription factor 1 (TCF-1), zinc-finger protein 10 (ZNF10/KOX1), and others that may affect cell growth. A deletion in chromosome 6 was also characterized Citation[40]. The two most obvious tumor suppressor genes in the latter locus, PLAGL1 and LATS1, however, showed normal sequences and normal gene expression. Furthermore, a copy number gain at 22q13 was found recurrent in 30% of AdCC in a large material Citation[41]. Use of DNA copy number changes for prognostication in AdCC did not seem promising due to a poor correlation between genomic alterations and tumor behavior (Jee KJ, Heikinheimo K, Makitie A, Knuutila S, Leivo I. Personal communication).
In recent years, it has become increasingly acknowledged that mucoepidermoid carcinomas are characterized by a t(11;19)(q21;p13) translocation Citation[7], Citation[8], Citation[42], Citation[43]. The resultant fusion gene is called MECT1-MAML2 (MECT1 is also called CRTC1, TORC1 or WAMTP1), in which a CREB coactivator is fused to the Notch coactivator MAML2. The fusion gene was found in more than half of mucoepidermoid carcinomas, and the fusion protein is expressed in all the three cell types of MEC Citation[9]. Analyses of potential downstream target genes revealed different expression of the cAMP/CREB genes (FLT1 and NR4A2) and the Notch genes (HES1 and HES5) in fusion-positive and fusion-negative MEC Citation[9]. Interestingly, underexpression of a CREB-binding protein gene in MEC was observed in expression arrays Citation[37]. MECT1-MAML2 fusion-positive patients had significantly less local recurrences, metastases, and tumor-related death compared to fusion-negative patients. Median survival for fusion-positive patients exceeded ten years while for fusion-negative patients it was 1.6 years. This suggests that the MECT1-MAML2 fusion is a useful tool for prognostication of MEC Citation[9]. Other recent whole-genome surveys using fine-resolution array CGH indicate that low-grade MEC shows no or minimal copy number gains and losses, while high-grade tumors display a number of extensive amplifications and deletions (Jee KJ, Heikinheimo K, Makitie A, Knuutila S, Leivo I. Personal communication). Indeed, the genomic changes in low-grade MEC and high-grade MEC shared no similarities.
Recently, combinations of genetic and epigenetic alterations have been reported in salivary gland carcinomas Citation[44]. Changes in methylation and loss of heterozygosity (LOH) in INK4a/ARF, RB1, p21, p27, PTEN, p53, MDM2 and O6-MGMT genes, and changes in the acetylation status of histone proteins H3 and H4 were studied. Methylation of RB1 gene was the most frequent, and seen in over 40% of cases. RB1 methylation correlated significantly with loss of RB1 protein in tumors, and a poor prognosis of the patients. LOH in RB1 gene was seen in 30% of cases. Acetylation of histones H3 and H4 was detected in 15–20% of tumors. It appears that epigenetic events, particularly silencing of tumor suppressor gene RB1 by promoter hypermethylation, as well as changes in histone acetylation may play roles in salivary gland carcinogenesis.
Conclusions
The small number of similarly deregulated genes in the major types of salivary gland carcinoma suggests heterogenic mechanisms of tumorigenesis. This is consistent with the great histopathologic diversity among carcinomas of salivary glands Citation[2]. Diversity is also implicated by tumor-type specific clustering of the studied epithelial carcinomas, and quite dissimilar patterns of genomic changes in high- and low-grade MEC and AdCC. Due to distinct translocations, non-overlapping amplifications, deletions and cell proliferation activities, and remotely similar histomorphologies, one may indeed question whether high-grade MEC and low-grade MEC represent the same tumor entity.
The small number of jointly overexpressed genes by major salivary gland carcinomas relate to cell adhesion, motility and shape (FN1, BGN, HXB), cell growth, metastasis, tumor angiogenesis and apoptosis (TIMP-1, IGFBP-5). The jointly underexpressed genes related to cell-cycle proteins, proteins of signal transduction and translation, and may be more directly involved in neoplastic cell growth. Interestingly, the jointly underexpressed CREBBP and MADH4/SMAD4 are intimately involved in the TGF-β transcription pathway of growth inhibition. MADH4/SMAD4 was also deleted or mutated in half of pancreatic carcinomas Citation[45]. Thus, overexpression of TIMP1, PLAT and SFN Citation[45], and underexpression of MADH4/SMAD4 are shared by carcinomas of the salivary glands and the pancreas, but not with other exocrine carcinomas. In salivary glands, a coordinated loss of SMAD4 and CREBBP functions could impair growth control and promote oncogenic transformation.
This work was supported by grants from the Finnish Cancer Society, Helsinki University Hospital Funds, Finska Läkaresällskapet and Maritza and Reino Salonen Foundation. Dr. Roderick HW Simpson is acknowledged for sharing his extensive knowledge on salivary gland pathology with the author.
References
- Seifert G. World Health Organization international histological classification of tumours: Histological typing of salivary gland tumours2nd ed. Springer, Berlin, Heidelberg, New York 1991
- Barnes L, Eveson JW, Reichart P, Sidransky D. Pathology and genetics of head and neck tumours. World Health Organization Classification of Tumours. IARC Press, Lyon 2005; 1–430
- Luukkaa H, Klemi P, Leivo I, Koivunen P, Laranne J, Makitie A, et al. Salivary gland cancer in Finland 1991–96: An evaluation of 237 cases. Acta Otolaryngol 2005; 125: 207–14
- Seifert G. Mucoepidermoid carcinoma in a salivary duct cyst of the parotid gland: Contribution to the development of tumours in salivary gland cysts. Pathol Res Pract 1996; 192: 1211–7
- Handra-Luca A, Lamas G, Bertrand JC, Fouret P. MUC1, MUC2, MUC4, and MUC5AC expression in salivary gland mucoepidermoid carcinoma: Diagnostic and prognostic implications. Am J Surg Pathol 2005; 29: 881–9
- Foschini M, Scarpellini F, Gown AM, Eusebi V. Differential expression of myoepithelial markers in salivary, sweat and mammary glands. Int J Surg Pathol 2000; 8: 29–37
- Enlund F, Behboudi A, Andrén Y, Öberg C, Lendahl C, Mark J, et al. Altered Notch signaling resulting from expression of a WAMP1-MAML2 gene fusion in mucoepidermoid carcinomas and benign Warthin′s tumors. Exp Cell Res 2004; 292: 21–8
- Martins C, Cavaco B, Tonon G, Kaye FJ, Soares J, Fonseca I. A study of MECT1-MAML2 in mucoepidermoid carcinoma and Warthin's tumor of salivary glands. J Mol Diagn 2004; 6: 205–10
- Behboudi A, Enlund F, Winnes M, Andren Y, Nordkvist A, Leivo I, et al. Molecular classification of mucoepidermoid carcinomas-Prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosomes Cancer 2006; 45: 470–81
- Goode RK, Auclair PL, Ellis AL. Mucoepidermoid carcinoma of the major salivary glands: Clinical and histologic analysis of 234 cases with evaluation of grading criteria. Cancer 1998; 82: 1217–24
- Ellis GL, Auclair PL. Malignant epithelial tumors. In: Atlas of tumor pathology, 3rd series, fascicle 17: Tumors of the salivary glands Washington DC: AFIP; 1996.
- Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, et al. Mucoepidermoid carcinoma: A clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol 2001; 25: 835–45
- Skálová A, Lehtonen H, von Boguslawsky K, Leivo I. Prognostic significance of cell proliferation in mucoepidermoid carcinoma of the salivary gland: Clinicopathological study using MIB1 antibody in paraffin sections. Hum Pathol 1994; 25: 929–35
- Heikinheimo AK, Laine MA, Ritvos OV, Voutilainen RJ, Hogan BLM, Leivo IV. Bone morphogenetic protein-6 is a marker of serous acinar cell differentiation in normal and neoplastic human salivary gland. Cancer Res 1999; 59: 5815–21
- Skálová A, Leivo I, von Boguslawsky K, Saksela E. Cell proliferation correlates with prognosis in acinic cell carcinomas of salivary gland origin. Immunohistochemical study of 30 cases using the MIB1 antibody in formalin-fixed paraffin sections. J Pathol 1994; 173: 13–21
- Hellquist HB, Sundelin K, Di Bacco A, Tytor M, Manzotti M, Viale G. Tumour growth fraction and apoptosis in salivary gland acinic cell carcinomas. Prognostic implications of Ki-67 and bcl-2 expression and of in situ end labelling (TUNEL). J Pathol 1997; 181: 323–9
- Brandwein MS, Jagirdar J, Patil J, Biller H, Kaneko M. Salivary duct carcinoma (cribriform salivary carcinoma of excretory ducts): A clinicopathologic and immunohistochemical study of 12 cases. Cancer 1990; 65: 2307–14
- Henley JD, Seo IS, Dayan D, Gnepp DR. Sarcomatoid salivary duct carcinoma of the parotid gland. Hum Pathol 2000; 31: 208–13
- Simpson RHW, Prasad A, Lewis JE, Skálová A, David L. Mucin-rich variant of salivary duct carcinoma: A clinicopathological study of four cases. Am J Surg Pathol 2003; 27: 1070–9
- Nagao T, Gaffey TA, Visscher DW, Kay PA, Minato H, Serizawa H. Invasive micropapillary salivary duct carcinoma: A distinct histologic variant with biologic significance. Am J Surg Pathol 2004; 28: 319–26
- Moriki T, Ueta S, Takahashi T, Mitani M, Ichien M. Salivary duct carcinoma: Cytologic characteristics and application of androgen receptor immunostaining for diagnosis. Cancer (Cancer Cytopathol) 2001; 93: 344–50
- Skálová A, Starek I, Vanecek T, Kucerova V, Plank L, Szepe P, et al. Expression of HER-2/neu gene and protein in salivary duct carcinomas of parotid gland as revealed by immunofluorescence in situ hybridization and immunohistochemistry. Histopathology 2003; 42: 1–9
- Brandwein-Gensler M, Hille J, Wang BY, Urken M, Gordon R, Wang LJ, et al. Low-grade salivary duct carcinoma: Description of 16 cases. Am J Surg Pathol 2004; 28: 1040–4
- Nagao K, Matsuzaki O, Saiga H, Sugano I, Shigematsu H, Kaneko T, et al. Histopathologic studies on carcinoma in pleomorphic adenoma of the parotid gland. Cancer 1981; 48: 113–21
- Di Palma S, Pilotti S, Rilke F. Malignant myoepithelioma of the parotid arising in a pleomorphic adenoma. Histopathology 1991; 19: 273–5
- Alós LL, Cardesa A, Bombí JA, Mallofré C, Cuchi A, Traserra J. Myoepithelial tumors of salivary glands: A clinicopathologic, immunohistochemical and flow-cytometric study. Semin Diagn Pathol 1996; 13: 138–47
- Savera AT, Sloman A, Huvos AG, Klimstra DS. Myoepithelial carcinoma of the salivary glands: A clinicopathologic study of 25 patients. Am J Surg Pathol 2000; 24: 761–74
- Reis-Filho JS, Simpson RHW, Fulford LG, Steppeler Y, Martins A, Schmitt FC. p63 distribution in normal salivary gland and salivary gland neoplasms (Abstract). Mod Pathol 2004; 17: 231A
- Nagao T, Sugano I, Ishida Y, Tajima Y, Matsuzaki O, Konno A, et al. Salivary gland malignant myoepithelioma: A clinicopathologic and immunohistochemical study of ten cases. Cancer 1998; 83: 1292–9
- Skálová A, Simpson RHW, Lehtonen H, Leivo I. Assessment of proliferative activity using the MIB1 antibody helps to distinguish polymorphous low grade adenocarcinoma from adenoid cystic carcinoma of salivary glands. Pathol Res Pract 1997; 193: 695–703
- Evans HL, Luna MA. Polymorphous low-grade adenocarcinoma: A study of 40 cases with long-term follow up and evaluation of the importance of papillary areas. Am J Surg Pathol 2000; 24: 1319–28
- Parrett TJ, Prasad AR, Raslan WF, Kakar S, Lewis JE. Long term and life-long follow-up in polymorphous low grade adenocarcinoma (Abstract). Mod Pathol 2002; 15: 223A
- Michal M, Skálová A, Simpson RHW, Raslan WF, Čuřík R, Leivo I, et al. Cribriform adenocarcinoma of the tongue: A hitherto unrecognized type of adenocarcinoma characteristically occurring in the tongue. Histopathology 1999; 35: 495–501
- Milchgrub S, Gnepp DR, Vuitch F, Delgado R, Albores-Saavedra J. Hyalinizing clear cell carcinoma of the salivary gland. Am J Surg Pathol 1994; 18: 74–82
- Frierson HF, El-Naggar AK, Welsh JB, Sapinoso LM, Su AI, Cheng J, et al. Large scale molecular analysis identifies genes with altered expression in salivary adenoid cystic carcinoma. Am J Surg Pathol 2002; 161: 1315–23
- Stallmach I, Zenklusen P, Komminoth P, Schmid S, Perren A, Roos M, et al. Loss of heterozygosity at chromosome 6q23-25 correlates with clinical and histologic parameters in salivary gland adenoid cystic carcinoma. Virchows Arch 2002; 440: 77–84
- Leivo I, Jee KJ, Heikinheimo K, Laine M, Ollila J, Nagy B, et al. Characterization of gene expression in major types of salivary gland carcinoma with epithelial differentiation. Cancer Genet Cytogenet 2005; 156: 104–13
- Gibbons MD, Manne U, Carroll WR, Peters GE, Weiss HL, Grizzle WE. Molecular differences in mucoepidermoid carcinoma and adenoid cystic carcinoma of the major salivary glands. Laryngoscope 2001; 111: 1373–8
- Rutherford S, Hampton GM, Frierson HF, Moskaluk CA. Mapping of candidate tumor suppressor genes on chromosome 12 in adenoid cystic carcinomas. Lab Invest 2005; 85: 1076–85
- Rutherford S, Yu Y, Rumpel CA, Frierson HF, Jr, Moskaluk CA. Chromosome 6 deletion and candidate tumor suppressor genes in adenoid cystic carcinoma. Cancer Lett 2005;Jul 27 (Epub ahead of print).
- Freier K, Flechtenmacher C, Walch A, Ohl S, Devens F, Burke B, et al. Copy number gains on 22q13 in adenoid cystic carcinoma of the salivary gland revealed by comparative genomic hybridization and tissue microarray analysis. Cancer Genet Cytogenet 2005; 159: 89–95
- Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet 2003; 33: 208–13
- Coxon A, Rozenblum E, Park YS, Joshi N, Tsurutani J, Dennis PA, et al. Mect1Maml2 fusion oncogene linked to the aberrant activation of cyclic AMP/CREB regulated genes. Cancer Res 2005; 65: 7137–44
- Kishi M, Nakamura M, Nishimine M, Ikuta M, Kirita T, Konishi N. Genetic and epigenetic alteration profiles for multiple genes in salivary gland carcinomas. Oral Oncol 2005; 41: 161–9
- Crnogorac-Jurcevic T, Efthimiou E, Nielsen T, Loader J, Terris B, Stamp G, et al. Expression profiling of microdissected pancreatic adenocarcinomas. Oncogene 2002; 21: 4587–94