Abstract
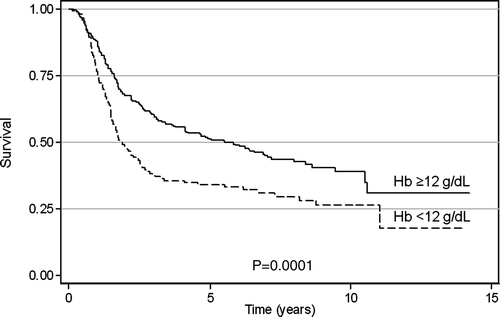
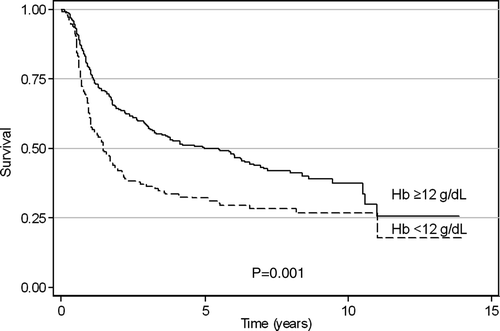
The prognostic impact of pretreatment hemoglobin (Hb) level and its changes during definitive radiotherapy was evaluated by univariate and multivariate analysis in the group of 453 FIGO IB–IIIB cervical cancer patients. Pretreatment anemia (Hb < 12 g/dl) was present in 148 patients (33%), and anemia at the end of irradiation in 48%; in 64% Hb level declined during therapy. Median overall survival in patients with initial Hb ≥12 g/dl was 66 months compared to 22 months in those with lower baseline Hb levels (p = 0.0001). This difference was mainly due to increased risk of distant spread in anemic patients (40% compared to 25% in subjects with pretreatment Hb ≥12 g/dl; p = 0.001). Baseline Hb ≥12 g/dl was also associated with longer disease-free survival and improved local control. Declining Hb level during radiotherapy predicted for impaired 5-year disease-free survival and local control probability. In multivariate analysis, low pretreatment Hb level remained associated with worse overall and disease-free survival, whereas adverse impact of declining Hb level on outcome was not observed. With regard to other clinical factors, stage and tumor extension (uni- or bilateral parametrium involvement for Stage III) were the only independent determinants of prognosis.
Anemia is one of the most common systemic symptoms accompanying cancer. Although the exact pathophysiologic mechanisms of cancer-related anemia are not fully understood, suggested reasons include changes in iron metabolism, suppression of erythroid progenitor cells by releasing tumor cytokines, impaired erythropoietin response on erythroid progenitor cells, and hemorrhage Citation[1]. The clinical impact of correcting Hb level during therapy by transfusions or recombinant human erythropoietin (rhEPO) administration remains to be determined Citation[2], Citation[3].
Adverse impact of low Hb levels in patients undergoing radiotherapy (RT) was demonstrated in various solid tumors including cervical cancer Citation[4–18]. It is still not determined whether the association of low Hb levels with outcome should be regarded as a real prognostic factor reflecting more aggressive biological behaviour of the tumor or as the result of a relative tumor radioresistance due to tumor hypoxia resulted by decreased oxygen-carrying capacity of the blood in case of anemia.
The association between low pretreatment Hb levels and poorer outcome in cervical cancer patients managed with RT has been demonstrated in a number of studies, however, only in some this Hb value was confirmed as an independent prognostic factor (). The impact of Hb changes during RT on its outcome in cervical cancer remains debatable Citation[6], Citation[9], Citation[10], Citation[12], Citation[14], Citation[15]. Decreased prognosis associated with declining Hb during RT was found in other malignancies and this effect was even stronger compared to pretreatment Hb values Citation[19].
Table I. Overview of selected studies investigating the prognostic significance of hemoglobin level in cervical cancer patients treated with definitive radiotherapy (pHb–pretreatment hemoglobin level).
In this study we analyzed the prognostic impact of baseline Hb levels and their changes during therapy in cervical cancer patients managed with definitive RT.
Materials and methods
The records of 453 consecutive patients with International Federation of Gynecology and Obstetrics (FIGO) stage IB–IIIB cervical cancer selected for definitive radiotherapy at the Department of Oncology and Radiotherapy, Medical University of Gdansk, Poland, between 1989 and 1999, were reviewed (). Apart from full history and gynecological examination, pretreatment evaluation included a complete blood cell count and chest X-ray. Most patients underwent abdominal and pelvic ultrasound. Computed tomography of the pelvis and abdomen or pelvic magnetic resonance imaging were performed occasionally, and cystoscopy and rectoscopy only if clinically indicated. After the completion of treatment, all patients were followed up by a radiation oncologist.
Table II. Patient characteristics (n = 453).
Patient and treatment data were analyzed retrospectively. Data on disease status and treatment complications were withdrawn from outpatient records. Full follow-up data were obtained for 419 patients (93%) and for the remaining 34 patients only the date of death was available. If symptoms caused by radiation could not be clearly sorted out from those of pelvic tumor progression, the data were recorded as missing. Recurrences, as established at their first occurrence, were divided into two categories: local (including vaginal relapse or relapse within pelvic radiation fields) and distant.
RT consisted of whole pelvis external beam RT (EBRT), 1.8–2.4 Gy per fraction, five days a week, to the International Commission on Radiation Units and Measurements (ICRU) reference dose of 19.4–58.8 Gy (median, 21.8 Gy). Additionally, 4.0–38.0 Gy (median, 18 Gy) boost to the parametrium, with a 4-cm-wide central block shielding of the brachytherapy (BRT) area was performed in 400 patients (88%). EBRT was accompanied by low-dose/medium-dose-rate 137Cs intracavitary BRT applications of 10–20 Gy at point A each administered with the use of standard Selectron (Nucletron, The Netherlands) applicators (consecutive insertions were performed at 10 days apart). Patients with early stage disease were usually managed with three BRT applications preceding EBRT, and those with locally advanced disease were administered one or two BRT applications during EBRT (the first insertion was performed after a dose of 20–40 Gy and the second — most frequently at the end of irradiation). The total ICRU reference dose defined as the sum of the EBRT dose in the ICRU reference point and BRT dose in the reference point (point A) was of 58–97.6 Gy (median, 81.5 Gy). The median overall treatment time was 58 days (range, 32–120 days). EBRT was applied with either two parallel opposite pelvic AP-PA fields (395 patients, 87%), four-field (box) technique (21 patients, 5%) or both techniques in sequence (36 patients, 8%). Standard pelvic fields, using 9 MeV linear accelerator or 60Co unit were used. Two small blocks shielding upper corners of the anterior-posterior fields were used in all patients. None of the patients included in this analysis (except for three patients administered low doses of nitrogen mustard) received primary or concomitant chemotherapy. Blood transfusions were given to 19 patients (4.2%). Recombinant rhEPO was not used.
Patient data base was updated on December 31, 2003. The time to event was measured from the first day of radiotherapy. Recurrences were diagnosed according to their localization, using the most appropriate methods. Vaginal and peripheral lymph node involvement in virtually all instances was confirmed histologically, as opposed to other relapse sites which in some patients were diagnosed based on imaging studies. For example, apparent radiological lung lesions were not subjected to biopsy and peri-aortic lymph nodes were considered involved if at least 1 cm in the longest diameter lymph node enlargement appeared on CT. Patients without disease and lost to follow-up, or patients dying of intercurrent disease were censored at the time of the event. Statistical analysis of survival was performed on three subsets: IB, IIA and IIB, and IIIA and IIIB. Analyzed variables included age (≥65 vs. <65 years), FIGO stage, grade, parametrial involvement (uni-, bilateral) in stage III, presence of hydronephrosis, number of BRT applications and overall treatment time. A threshold Hb level of 12 g/dl for anemia was used. Scoring included pretreatment Hb level and its relative changes (pretreatment Hb vs Hb recorded during the last week of irradiation. Categorical and continuous variables were used wherever appropriate. Tumor size was recorded only in selected patients; therefore this variable was not included in the analysis. Survival curves were calculated according to the Kaplan-Meier method and compared with log-rank test. For multivariate analysis, a Cox backward stepwise regression model was used. P-value of < 0.05 was considered statistically significant.
Results
Hb level
The mean pretreatment and final Hb levels were 12.3 g/dl±1.6 and 11.9 g/dl±1.3, respectively. Three hundred and five patients (67%) presented with a normal baseline Hb (≥12 g/dl), 59 (13%) had mild anemia (Hb 11–11.9 g/dl), and the remaining 89 patients (20%) had moderate to severe anemia (Hb < 11g/dl). The corresponding numbers of patients with respective final Hb levels were 184 (52%), 92 (26%) and 80 (22%). Hb value at the end of therapy was available for 356 patients (79%), of whom in 172 (48%) Hb was <12 g/dl. Overall, in 227 patients (64%) Hb value declined at the end of therapy, of whom in 63 (28%) less than 5%, in 57% in a range of 5–15% and in 34 (15%) more than 15%. In 117 of patients (34%) Hb increased during therapy, of whom in 69 (59%) by < 10% of the initial value, in 33 (28%) by 10–30%, and in the remaining 15 (13%) by > 30%.
+Impact of Hb level on treatment outcome
The 5-year overall survival (OS) was 44%; 71% for Stage IB, 60% for Stage II, and 28% for Stage III. The respective 5-year disease-free survival (DFS) probabilities were 70%, 59%, and 26%. At a median follow-up of 4.4 years (range, 4–10 years) 88 of 419 assessable patients (21%) relapsed within the pelvis, and 124 (30%) developed distant metastases of whom 97 (78%) — without local recurrence. On univariate analysis patients with baseline Hb ≥12 g/dl had significantly better OS than those with baseline Hb of <12 g/dl (median survival of 66.2 vs 22.2 months, respectively, p = 0.0001) . Furthermore, initial Hb level of ≥12 g/dl was also predictive for longer DFS and improved local control (LC) (p = 0.001 and 0.0043, respectively). Distant metastases occurred in 25% and 40% of patients with baseline Hb ≥12 g/dl and < 12 g/dl, respectively (p = 0.001). A relative decline of Hb level was predictive for DFS (p = 0.0132) and for LC (p = 0.0222), but not for OS (p = 0.262). In multivariate Cox analysis, pretreatment Hb level was the second (after stage of disease and parametrial tumor extension in Stage III), predictive factor for OS and the first for DFS (), whereas its effect on LC was at the borderline level (p = 0.057). Relative changes of Hb values during RT did not significantly affect survival and LC.
Table III. The impact of particular factors on overall survival (OS), disease-free survival (DFS), local control (LC) and distant control (DC): multivariate analysis.
Discussion
In this series 33% of patients presented with anemia (<12 g/dl), and in 66% Hb level declined during RT. Overall, anemia persisted throughout therapy in 48% of cases. Disease-related anemia is a common hematological abnormality accompanying cervical carcinoma Citation[1], Citation[3], Citation[10]. Decreased Hb level both prior to and during treatment was reported to be associated with other tumor-related poor prognostic factors, such as advancing tumor stage and “bulky” disease Citation[3], Citation[10].
In this study of cervical cancer patients applied definitive RT pretreatment Hb level below 12 g/dl was associated with remarkably shorter OS compared with non-anemic patients. In the multivariate analysis initial Hb remained an independent prognostic factor for both OS and DFS. An independent impact of baseline Hb level on both survival and LC in cervical cancer was demonstrated in a series of studies Citation[6], Citation[9], Citation[10], Citation[12], Citation[15], in which definitions of anemia varied from below 10 g/dl to below 12.5 g/dl. However, in some studies, prognostic value of Hb level was not confirmed in multivariate analysis Citation[6], Citation[9], Citation[10], Citation[12].
In this series baseline Hb < 12 g/dl was associated with increased risk of local relapse at the borderline level of significance. Several reasons might have attenuated the association between pretreatment anemia and the risk of pelvic failure in our material: these include potential underreporting of some recurrences (data were collected retrospectively) and other methodological issues. Prognostic impact of pretreatment anemia might have also been modified by therapy (vaginal bleeding discontinuation), and in some patients-by correcting anemia with transfusions. In patients with “bulky” tumor the effect of initial anemia might have been diminished due to strong impact of tumor size on the efficacy of RT. Some authors Citation[3] reported that anemia does not exert an independent prognostic impact but represents only an epiphenomenon linked to known adverse prognostic factors such as tumor advancement. Finally, our findings may represent a no linear relationship between Hb levels, radiation response, and other tumor-related prognostic factors contributing to LC and survival Citation[6]. Interestingly, in the Canadian survey significant reduction in the distant failure risk, independent of a reduction in the pelvic failure was demonstrated in cervical cancer patients with initial normal or increasing Hb level Citation[10]. More frequent distant failure in patients with initial Hb < 12g/dl compared to non-anemic patients (25% and 40%, respectively) was also shown in our series.
Tumor hypoxia is considered a possible and attractive radiobiological explanation for increased failure rates in anemic patients, including those with cervical cancer. Hypoxia is known to mediate molecular changes related to cellular processes which may result in increased spontaneous aggressiveness through clonal selection and genomic changes, increased tumor angiogenesis, and relative tumor resistance to therapy. According to some authors hypoxia-induced treatment failure in advanced squamous cell carcinoma of the cervix is primarily caused by hypoxia-induced radiation resistance rather than hypoxia-induced metastases Citation[20]. However, recent data indicate that the relationships between anemia and tumor hypoxia (as well as the effect of transfusion) in patients with cancer are much more complex than considered initially Citation[3], Citation[21]. Of note, some authors reported that decreased progression-free survival in hypoxic cervical cancers was related to an increased risk of distant metastases rather than to an effect on pelvic control Citation[22].
With regard to other clinical factors in this series, stage and tumor extension (uni- or bilateral parametrium involvement for Stage III) were the only independent determinants of prognosis in a multivariate analysis. Moreover, the age ≥65 years was associated with lower risk of local and distant relapse.
In some studies the change of Hb level during radiotherapy was the strongest prognostic factor for LC and survival in cervical cancer Citation[6], Citation[9], Citation[10], Citation[12]. In this series relative changes of pretreatment Hb did not significantly affect survival and LC. There is evidence though that a simple comparison of initial and final Hb levels (as we had sufficient data on Hb levels during RT in only a number of patients) does not reflect precisely Hb levels throughout treatment. Furthermore, a decline from baseline normal Hb may have a different prognostic impact compared to a decline from moderate or severe anemia. Grogan et al. Citation[10] in a study of 605 patients with stage IB–IVA cervical cancer managed with definitive radiotherapy (≥35 Gy total dose, with or without concurrent chemotherapy) demonstrated on univariate analysis significantly better survival probabilities, DFS and LC for patients with baseline Hb ≥12 g/dl compared to those with lower Hb levels. On multivariate analysis, baseline Hb was no longer significant, but average weekly nadir Hb (AWNH) levels (calculated by averaging the weekly nadir Hb) during RT was shown to be the second (after tumor stage) most important predictor of survival. In that study favorable survival was attributed to AWNH level of ≥12 g/dl, regardless of whether baseline Hb was below or above this value (5-year survival probability of 70% and 74%, respectively). Patients with higher AWNH had also significantly lower risk of local and distant relapse. The worst prognosis was seen in patients with a high initial Hb level that fell off during RT. Authors proposed that correction of anemia (by transfusion, to Hb level of ≥12 g/dl during RT) abrogated the adverse effect of preexisting anemia. Similarly, in Girinski et al. Citation[9] study multivariate analysis revealed that Hb level was prognostic only during treatment and not before. Patients who had at least one Hb value <10 g/dl during therapy had twice the risk of locoregional failure compared with patients in whom all Hb measurements were >10 g/dl. In another study including cervical cancer patients poor OS and LC was associated with the midtherapy (at 19.8 Gy) Hb level of <11 g/dl, regardless of initial Hb values Citation[6].
From a radiation oncologist's point of view the two key questions remain: could correction of anemia during radiation (by blood transfusion or erythropoietin administration) overcome the negative prognostic effect of low Hb level and what is the optimal Hb value that should be maintained during RT.
The results of the only randomized trial addressing the benefit of blood supplementation in cervical cancer patients seem to be inconclusive Citation[3]. Recent experimental data suggest that transfusion cannot reduce the fraction of radiation-resistant hypoxic tumor cells extensively; as only 50% of patients with cervical cancer demonstrated an increase in tumor oxygenation following transfusion Citation[23]. Improved pelvic control and a trend towards increased DFS but no effect on metastases-free survival were demonstrated in the study assessed the response to routine blood transfusion (before or during RT; in cases with Hb level ≤11 g/dl) Citation[12]. Others reported transfusion during treatment as an adverse prognostic factor for survival and local control Citation[7], Citation[9], Citation[24]. Santin et al. Citation[24] suggested that routine blood transfusion of anemic cervical cancer patients does not improve an outcome and may represent an independent variable predictive of diminished survival after RT. In our series, following to the departmental policy, only a few symptomatic patients with severe anemia were transfused during therapy.
Introduction of rhEPO has provided an alternative to blood transfusions in the management of anemic cancer patients. This therapy may have a positive effect on tumor response Citation[25], however two randomised studies demonstrated its deleterious effect on survival Citation[26], Citation[27]. These findings call for a cautious use of rhEPO, particularly in advanced disease.
It has been suggested that Hb levels of 12–14 g/dl are optimal for tumor oxygenation Citation[21]. Nevertheless, in Grogan et al. Citation[10] study Hb levels ≥13 g/dl were not associated with additional gains in survival. Importantly, in that study the survival benefit associated with maintaining Hb level at ≥12 g/dl was caused by both improved pelvic control and a reduction in distant metastatic disease.
Recently, chemo-radiation (CRT) using cisplatin has become the standard of care in locally advanced cervical cancer. This management may further worsen anemic status. Indeed, greater anemia in the CRT group compared to RT alone (for acute toxicity grade 3 and 4: OR 1.63, 95%, CI 1.09–2.46) was demonstrated in a systematic review of concomitant therapy for cervical cancer Citation[28]. Additionally, the effect of anemia on the pharmacokinetics of cytostatics is still a subject of investigation Citation[29]. According to Obermair et al. Citation[30] in cervical cancer patients applied CRT low nadir Hb (the lowest Hb during CRT) was highly predictive for shortened progression-free survival, whereas the Hb before treatment was not prognostically significant.
In summary, in this series baseline Hb level is an independent parameter of poor overall and disease-free survival in cervical cancer patients applied definitive irradiation. This effect is mainly due to increased risk of distant metastases.
References
- Mercadante S, Gebbia V, Marazzo A, Filosto S. Anemia in cancer: Pathophysiology and treatment. Cancer Treat Rev 2000; 26: 303–11
- Dusenberg KE, McGuire WA, Holt PJ, et al. Erythropoetin increases hemoglobin during radiation therapy for cervical cancer. Int J Radiat Oncol Biol Phys 1994; 29: 1079–84
- Fyles AW, Milosevic M, Pintilie M, Syed A, Hill RP. Anemia, hypoxia and transfusion in patients with cervical cancer: A review. Radiother Oncol 2000; 57: 13–9
- Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer. Cancer 2001; 91: 2214–21
- Chatani M, Matayoshi Y, Masaki N, Inoue T. High-dose rate intracavitary irradiation for carcinoma of the uterine cervix. Strahlenther Onkol 1999; 173: 379–84
- Dunst J, Kuhnt T, Strauss HG, et al. Anemia in cervical cancers; impact on survival, patterns of relapse, and association with hypoxia and angiogenesis. Int J Radiat Oncol Biol Phys 2003; 56: 778–87
- Fyles AW, Pintile M, Kirkbride P, Levin W, Manchul LA, Rawlings GA. Prognostic factors in patients with cervix cancer treated by radiation therapy: Results of a multiple regression analysis. Radiother Oncol 1995; 35: 107–17
- Gasinska A, Urbanski K, Adamczyk A, Pudelek J, Lind BK, Brahme A. Prognostic significance of intratumour microvessel density and haemoglobin level in carcinoma of the uterine cervix. Acta Oncol 2002; 41: 437–43
- Girinski T, Pejovic-Lenfant MH, Bouhis J, et al. Prognostic value of hemoglobin concentration and blood transfusions in advanced carcinoma of the cervix treated by radiation therapy: Results of a retrospective study of 386 patients. Int J Radiat Oncol Biol Phys 1989; 16: 37–42
- Grogan M, Thomas GM, Melamed I, et al. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer 1999; 86: 1528–36
- Hsu H-CH, Leung SW, Huang E-Y., et al. Impact of the extent of parametrial involvement in patients with carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 1998; 40: 405–10
- Kapp DS, Fisher D, Gutierrez E, Kohorn IE, Schwartz PE. Pretreatment prognostic factors in carcinoma of the uterine cervix: A multivariate analysis of the effect of age, stage, histology, and blood counts on survival. Int J Radiat Oncol Biol Phys 1983; 9: 445–55
- Karolewski K, Korzeniowski S, Sokolowski A, Urbanski K, Kojs Z. Prognostic significance of pretherapeutic and therapeutic factors in patients with advanced cancer of the uterine cervix treated with radical radiotherapy. Acta Oncol 1999; 38: 461–8
- Logsdon MD, Eifel PJ. FIGO IIIB squamous cell carcinoma of the cervix: An analysis of prognostic factors emphasizing the balance between external beam and intracavitary radiation therapy. Int J Radiat Oncol Biol Phys 1999; 43: 763–75
- Pedersen D, Sogaard H, Overgaard J, Bentzen SM. Prognostic value of pretreatment factors in patients with locally advanced carcinoma of the cervix treated by radiotherapy alone. Acta Oncol 1995; 34: 787–95
- Werner-Wasik M, Schmid CH, Bornstein L, Ball HG, Smith DM, Madoc JH. Prognostic factors for local and distant recurrence in Stage I and II cervical carcinoma. Int J Radiat Oncol Biol Phys 1995; 32: 1309–17
- Vigario G, Kurohara SS, George FW. Association of hemoglobin levels before and during radiotherapy with prognosis in uterine cervix cancer. Radiology 1973; 106: 649–52
- Yalman D, Aras AB, Ozkok S, et al. Prognostic factors in definitive radiotherapy of uterine cervical cancer. Eur J Gynecol Oncol 2003; 3: 309–14
- MacRac R, Shyr Y, Johnson D, Choy H. Declining hemoglobin during chemoradiotherapy for locally advanced non-small cell lung cancer is significant. Radiother Oncol 2002; 64: 37–40
- Rofstad EK, Sunfor K, Lyng H, Trope CG. Hypoxia-induced treatment failure in advanced squamous cell carcinoma of the uterine cervix is primarily due to hypoxia-induced radiation resistance rather than hypoxia-induced metastases. Br J Cancer 2000; 83: 354–9
- Vaupel P, Thews O, Mayer A, Hockel S, Hockel M. Oxygenation status of gynecologic tumors: What is the optimal hemoglonim level?. Strahlenther Onkol 2002; 178: 727–31
- Fyles BA, Milosevic M, Hedley D, et al. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. J Clin Oncol 2002; 20: 680–7
- Sundfor K, Lyng H, Kongsgard UL, Trope C, Rofstad EK. Polarographic measurement of pO2 in cervix carcinoma. Gynecol Oncol 1997; 64: 230–6
- Santin AD, Bellone S, Parrish RS, et al. Influence of allogenic blood transfusion on clinical outcome during radiotherapy for cancer of the uterine cervix. Gynecol Obstet Invest 2003; 56: 28–34
- Larsson A-M, Landberg G, Pahlman S, Albertsson M. Erythropoietin enhances response to treatment in patients with advanced breast cancer. Acta Oncol 2004; 43: 594–7
- Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet;362:1255–60.
- Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: A survival study. J Clin Oncol 2005; 23: 5960–72
- Kirwan JM, Symonds P, Creen JA, et al. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother Oncol 2003; 68: 217–26
- Van Belle S J-P, Cocquyt V. Impact of haemoglobin levels on the outcome of cancers treated with chemotherapy. Crit Rev Oncol/Hematol 2003; 47: 1–11
- Obermair A, Cheuk R, Horwood K, et al. Anemia before and during concurrent chemoradiotherapy in patients with cervical carcinoma: Effect on progression-free survival. Int J Gynecol Cancer 2003; 13: 633–9