Abstract
The number of elderly patients with cancer is steadily increasing in developed countries and their treatment is a growing challenge for oncological departments. Anal cancer is the first tumour in which chemoradiotherapy with the intent of organ preservation has largely replaced surgery and is an interesting model of modern multimodal oncological treatment. At the Department of Oncology of the Helsinki University Central Hospital we have treated all patients irrespective of age following the same guidelines if there have been no specific contraindications on the basis of intercurrent diseases. The results suggest that the chemoradiotherapy protocol used in the treatment of anal cancer is reasonably well tolerated in elderly patients and the tumour control is comparable to those achieved in younger patients. After successful cancer therapy the life expectancy in these patients can be very long.
The number of elderly people in developed countries has been steadily increasing during the past decades. In 1983 the average life-expectancy in females in Finland was 78.3 years and in males 70.2 years. In 2003 the corresponding figures were 81.8 and 75.1 years and in the year 2040 the expected figures are 86.3 and 82.1 years, respectively Citation[1]. In 2003 52% of new cancer diagnoses were made in patients aged 70 years or older, and this proportion is increasing Citation[2]. In many elderly people general health remains good until the final years of life and therefore decisions of cancer treatment are more dependent on the patient's general health than age.
Anal cancer is a rare tumour that represents 4% of all cancers of the lower gastrointestinal tract. In Finland about 20 new cases are diagnosed annually and 60% of the patients are women and 40% men Citation[2]. Anal cancer is, in spite of its rarity, an interesting example of modern oncological treatment. Anal squamous cell carcinoma is the first cancer in which organ preserving chemoradiotherapy has largely replaced surgery and is also the first tumour in which chemoradiotherapy was proved to be superior to radiotherapy alone. At present there is wide consensus that chemoradiotherapy is the treatment of choice especially in advanced anal cancer with surgery reserved for salvage therapy Citation[3], Citation[4]. The main advantage of non-surgical treatment is preservation of normal anal function and the tumour control figures achieved by chemoradiotherapy are as least as good as those in historical surgical series, although randomized studies directly comparing chemoradiotherapy and surgery are lacking. The pioneer study on chemoradiotherapy with the intent of organ preservation in the treatment of anal cancer was published by Nigro et al. in the 1970s Citation[5], whereafter the results have been confirmed in prospective, randomized trials Citation[6–8]. On the basis of these trials chemoradiotherapy has been widely accepted as standard therapy especially for advanced disease. The greatest benefit of chemoradiotherapy is achieved in large tumours. Nevertheless, in the UKCCCR trial this was also seen also in patients with T1-2N0 tumours Citation[6]. The best documented and most widely used concomitant chemotherapy schedule is the combination of mitomycin and fluorouracil given twice during the course of radiotherapy. The use of cisplatin instead of mitomycin is under investigation Citation[4]. The radiotherapy is usually given as external radiotherapy by shrinking field technique starting with the fields first encompassing the primary tumour and the locoregional nodal areas up to a total dose of 30–45 Gy, whereafter the primary tumour is boosted either by external RT or by interstitial radiotherapy Citation[4] yielding to a total dose of 50–55 Gy in 1.8–2.0 Gy fractions.
The multimodality oncological treatment schedule used in anal cancer offers a good opportunity to evaluate the efficacy and tolerability of cancer treatments in elderly patients. At the Department of Oncology of the Helsinki University Central Hospital we have been using the same chemoradiotherapy protocol in the treatment of anal cancer for the last 14 years. By now we have treated 62 patients with a follow-up of 24 months or more. If no specific contraindications to chemoradiotherapy on the basis of intercurrent diseases were observed, all age groups were treated following the same guidelines. Twenty-three of the patients were 70 years of age or older. We have now analyzed the results and compared the treatment outcome in patients ≥ 70 years to that achieved in younger patients. The effect of comorbidities on the treatment results in the elderly patients is also analyzed.
Materials and methods
Patients
This study is based on a cohort of patients diagnosed with primary anal cancer and treated with chemoradiotherapy in the Department of Oncology, Helsinki University Central Hospital, Finland between January 1992 and November 2003. During this period 86 new cases of squamous cell anal carcinomas were diagnosed in the district of Helsinki University Central Hospital. Eighty of these 86 patients (93%) were sent to oncologic consultation to the Department of Oncology. Sixty two of the 80 patients (77.5%) received chemoradiotherapy as primary treatment. The reasons for not giving chemoradiotherapy to the remaining 18 patients were primary radical surgery in five patients, another intercurrent malignant tumour in three patients and other serious intercurrent diseases considered to be a contraindication for chemotherapy in seven patients (recent myocardial infarct in three, cerebral infarct with total hemiparesis, Mb Alzheimer, renal insufficience requiring dialysis and AIDS, in one patient each). In only one case age (96 years) was the main reason for giving radiotherapy only.
Twenty-three of the 62 patients treated with chemoradiotherapy were at least 70 years old. The mean follow-up time for patients surviving is 72 months (range 24–168 months). Rectoscopy, computed tomography (CT), and since 2001 also pelvic MRI were used in clinical staging, which was done according to the International Union Against Cancer (UICC) tumour-node-metastasis (TNM) classification version 1997 Citation[9]. The main patient and tumour characteristics are presented in .
Table I. Patients and tumour characteristics.
A histological tissue biopsy was taken in all cases from the primary tumour and, when necessary, a fine needle biopsy was used to ascertain suspected nodal metastasis.
The amount of comorbidities and permanent medications in the elderly patients is presented in .
Table II. Comorbidities and permanent medications in elderly patients (n = 23).
Radiotherapy
The characteristics of the radiotherapy are presented in . Initially all patients were treated by 15 or 18 MV photons from linear accelerator with conventional 4-field arrangement. CT-based treatment planning (Cadplan®) was performed for all cases. Since 2003 intensity modulated radiotherapy (IMRT) with 6MV photons and 5–7 fields has been used as routine treatment in this patient group. The treatment plans for IMRT were generated using Helios® inverse planning software of the Cadplan® treatment planning system version 6.27. A polyurethane fixation system was used. The treatment volume encompassed the primary tumour and the regional lymph nodes (inguinal, perirectal and parailiacal nodes) up to the total dose of 39.6 Gy in 1.8 Gy daily fractions (PTV1). Total margins of 1 cm were used from clinical target volume (CTV) to PTV. In order to avoid excess treatment toxicity, the parailiacal nodes were treated only in cases with documented nodal metastasis at lower nodal levels. After the nodal fields had been finished an external radiotherapy booster of 5.4 Gy in 1.8 Gy fractions was given to the primary tumour (PTV2).
Table III. Radiotherapy.
After the total dose of 45 Gy a booster dose to the primary tumour (PTV3) was given either as external radiotherapy using a perineal electron treatment field (n = 29) or by interstitial brachytherapy (n = 37). The mean external RT booster dose was 8.8 Gy (range, 5.4–15 Gy). The electron energy used in perineal field booster therapy was estimated from the CT-scans, but as the patient position during electron field treatment is different from that in the CT scans, these fields were simulated separately. The brachytherapy booster was given with cesium 137 afterloader (Selectron LDR) up to a total dose of 20–30 Gy in two fractions and two weeks interval before the year 1996 (n = 15). After 1996 the brachytherapy has been given with iridium 192 afterloader (Microselectron HDR) in two 5–6 Gy weekly fractions (n = 22). Possible inguinal nodal metastases were boosted by separate electron fields and deeper situated nodal metastases by photon fields to a mean total dose of 9.9 Gy (range, 5.4–23.6 Gy).
Chemotherapy
All patients were scheduled to receive concomitant chemotherapy mitomycin (10 mg/m2 on days 1 and 29) and fluorouracil (1 g/m2/day on days 1–4 and days 29–32 given as continuous infusion). In patients with 70 years or more of age there were significantly more dose alterations (two full-dose cycles in 61% of the patients vs. 90% in the group under 70 years, p = 0.01). The cycles of concomitant chemotherapy given and causes of altered schedule are presented in .
Table IV. Alterations in chemotherapy schedule.
Scoring of treatment related acute and late toxicity
The acute treatment related toxicity (toxicity during the treatment and three months following the treatment) was scored according to the RTOG acute radiation morbidity scoring system Citation[10] during the treatment. The worst scores for each patient are reported. In evaluation of the late morbidity (treatment related toxicity occurring >3 months after the end of the treatment), the RTOG/EORTC late radiation morbidity scoring scheme was used Citation[10]. The occurrence of possible late toxicities was registered during every follow-up visit.
Follow-up of the patients
After chemo-radiotherapy the patients were followed in the Department of Oncology of the Helsinki University Central Hospital at three months intervals during the first two years and thereafter at six months intervals until five years. In addition, rectoscopy was performed 1–2 months following chemo-RT and thereafter every six months until five years in the Department of Gastrointestinal Surgery of the same hospital. Biopsies were taken from any suspicious tissue observed in rectoscopy.
Statistical analysis
The NCSS 2000 statistical software (NCSS Statistical Software, Kaysville, UT) was used for statistical calculations and graphical presentations. The cumulative survival was estimated with the Kaplan-Meier product-limit method and comparisons of the survival rate between groups were done using the log-rank test or univariate Cox regression analysis. Frequency tables were analyzed using either χ2 or Fisher's exact test. All p-values are 2-tailed. Life expectancy calculations are based on data available from the Statistics Finland (State Statistical Center).
Results
Loco-regional tumour control
Local control was primarily achieved in 59 of the 62 patients; two of the three treatment failures were in the older and one in the younger patient group. The patients aged > 70 years with primary treatment failure were 71 and 72 years of age and the primary tumours were staged T3N2 and T3N0, respectively. Both of these patients received full-dose radiotherapy but only one cycle of chemotherapy. The third patient was a 42 year old male with T4N2 primary tumour and the patient received full-dose chemo- and radiotherapy. During the follow-up period nine local recurrences were observed, 3/23 and 6/39, respectively. Thus the total amount of local failures was 12/62 (19%): for the older 5/23 (22%) and for the younger patient group 7/39 (18%). No statistical difference was observed (p = 0.79, log-rank test, p = 0.86, Cox's regression analysis when analysed as a continuous variable). The median time to local recurrence was nine (range 6–24) months. The local control figures in patients <70 years and ≥70 years are presented in , upper panel.
Figure 1. Upper panel: local control and time to local recurrence; lower panel: disease-free survival after chemoradiotherapy in patients over 70 years (thick grey line) and in patients less than 70 years (thin black line).
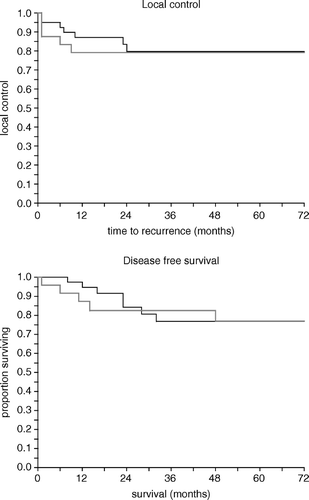
When analyzed for the whole patient cohort, the tumour stage was found to be a significant prognostic factor (p = 0.03). There was also a trend towards lower tumour control probability with growing primary tumour size and nodal metastasis (p = 0.22 and p = 0.06, respectively). The tumour histology was found to be non-significant (p = 0.31).
There was also some tendency towards inferior local control with increasing total treatment time, but this did not reach statistical significance (p = 0.23). The time from external radiotherapy to brachytherapy booster was found to be non-significant (p = 0.83). There were fewer local recurrences in patients with T3-4 tumours treated with brachytherapy booster than in those treated with external radiotherapy: 2/15 (13%) vs. 3/9 (33%), p = 0.24, χ2 statistics.
Seven patients were treated by abdominoperineal resection (APR) for local recidual (n = 3) or recidive (n = 4) tumour. All three of the patients with primary failure after chemoradiotherapy also failed after APR. In contrast, three of the four patients undergoing APR for local recidive after successful primary chemoradiotherapy were salvaged by surgery; the mean follow-up time for these patients is now 69 months (range 17–135 months). After APR delayed wound healing demanding plastic surgery was reported in one patient.
Disease free survival
Disease free survival (DFS) in the whole patient cohort was 84% at two years and 77% at five years. Six patients (9.7%) developed distant metastasis during the follow-up. The median time from chemoradiotherapy to metastasis was 11 months (range, 4–30 months). No local or distant recurrences were observed after three years of follow-up. The DFS in patients less than 70 and ≥70 years of age is presented in , lower panel. No difference was observed between the age groups (p = 0.81, log-rank test, p = 0.79 Cox's regression analysis). The tumour stage and nodal status were the only factors found to be of statistical significance for DFS (p = 0.07 and 0.05, respectively).
Effect of comorbidities on treatment result and life expectancy following chemoradiotherapy in elderly patients
The number of comorbidities and permanent medications in elderly patients at the time of the treatment was analyzed. The diagnoses of comorbidities in patients ≥ 70 years is presented in the first column of , the number of comorbities per patient in the second column and the number of permanent medications per patient in the third column. In patients with no intercurrent diseases (n = 10), one local failure (10%) was observed and in patients with comorbidities, the amount of local failures was 4/13 (31%). In patients with intercurrent diseases, there were more dose reductions during the chemotherapy course (1/10 vs 6/13, p = 0.09). A trend towards inferior local control and DFS was observed in patients with dose reductions of chemotherapy (p = 0.12 and p = 0.11, log-rank test, respectively). The same observation was made also with permanent medications. However, as excess medication is a consequence of comorbidities, no independent statistical significance can be counted for it.
The mean age of the elderly patients at the time of diagnosis was 77 years (range 70–89 years). Four of the patients cured for cancer have died for intercurrent diseases at the mean age of 85 years (range 81–89 years). Thirteen of the patients of ≥70 years at diagnosis are still alive and the mean age of these patients at the time of this analysis is 83 years (range 74–94 years). On the basis of life expectancy tables these patients can be expected to reach the mean age of 90 years (range 85–95 years).The life expectancy calculations are made according to the data obtained from Statistics Finland Citation[1].
Treatment related acute toxicity
The acute adverse effects are presented in . Some degree of rectal irritation presented as diarrhoea of varying degrees was observed in every patient. Diarrhoea tended to be more severe in the older patients (grade 3–4 48% vs 21%, p = 0.04) and a trend towards more frequent 5-fluorouracil-related cardiac toxicity and stomatitis was also observed (p = 0.14 and p = 0.09, respectively). Skin and mucosal eruption around the anal region were observed in every patient and there was no difference in severity between the age groups. The chemotherapy-related haematological toxicity was also similar irrespective of the age. Brachytherapy was well tolerated in both age groups and no acute complications attributable to interstitial radiotherapy occurred.
Table V. Chemoradiotherapy-related grade 3–4 acute toxicity.
Treatment related late toxicity
Radiation proctitis was the most frequent late side effect following chemoradiotherapy. The proctitis was, however, mild in most of the patients. In patients less than 70 years of age four cases of grade 2 and four of grade 3 proctitis were observed (8/39 = 21%) and in the patients ≥ 70 years the corresponding figures were three and two cases (5/23 = 22%). The difference was statistically insignificant; p = 0.64, χ2 statistics. No g-i complications attributable to radiation strictures or fistules were observed. None of the patients underwent abdominoperitoneal resection for late bowel complications. Skin reactions around the anal region were also observed, especially in patients treated by perineal booster field. There was no difference in skin reactions between the age groups (p = 0.72, χ2 statistics). No chronic skin ulcerations were observed and the most severe forms of skin reactions were skin atrophy and teleangiaectasia at anal region. One case of grade 3 radiation cystitis was observed in the older patient group.
Discussion
In the present study the local control and disease free survival achieved by chemo-radiotherapy were identical in elderly and younger patients. In the whole cohort of patients nodal status and the tumour stage were observed to be of prognostic significance. There was a trend towards better local control in T3-4 tumours when treated with brachytherapy booster. This difference did not, however, reach statistical significance. The achieved tumour control figures are good as compared with those from randomized clinical studies Citation[6], Citation[8]. Chemotherapy course alterations were more often needed in the older age group and an excess of 5-fluorouracil associated stomatitis and cardiac toxicity was observed in these patients. Also in chemoradiotherapy associated diarrhoea there was a significant difference. The lack of any difference in haematological toxicity is probably due to the more frequent dose reductions in the older age group. A trend towards inferior local control and DFS was observed in elderly patients with dose reductions of chemotherapy. Moreover, in two of the three primary treatment failures the patients had received only one cycle of chemotherapy. No difference in treatment related late toxicity was observed.
Several factors restricting aggressive, curative-intent cancer therapy in elderly patients have been recognized. As consequence of age, the tolerance to chemotherapy may be altered as a consequence of altered pharmacokinetics, pharmacodynamics and decreased tolerance of normal tissues to cytotoxic therapy Citation[11], Citation[12]. Even more important than actual age of the patients seems to be the existence of comorbidities Citation[12], Citation[13]. It has been calculated that the average number of diseases in persons aged 77 is 3.7 Citation[14]. These comorbidities and also the medications necessary for their treatment must be taken into consideration in cancer treatment. Thus, before making decisions on treatment of cancer, a careful analysis of the patient's general health is warranted. In a review by Gosney assessment scales possibly useful in pretreatment evaluation of elderly patients with cancer are presented Citation[15].
In clinical cancer trials elderly patients are often excluded, because they are considered to be at risk of increased treatment related toxicity and because in many clinical trials life expectancy of included patients must be over 10 years. In recent years several studies have been published on outcome of cancer treatments in elderly patients. In a study by Bernardi et al. Citation[16] on head and neck cancers the results from several studies on radiotherapy, surgery, chemotherapy and combined modality therapy in aged patients were analysed. It was concluded that radiotherapy is a feasible treatment in elderly patients, also in very advanced age groups. It was also stressed that elderly patients cannot be excluded from chemoradiotherapy programs of organ preservation and patients aged 70–79 without severe comorbidities must be treated in the same exact manner as younger patients, but supportive treatment must be increased. In a retrospective review of 273 cases on head and neck cancers in elderly patients it was concluded that when properly monitored, conventional therapies seem feasible in older patients Citation[17]. Same results have been achieved from studies on treatment of gastrointestinal cancers. In a study on preoperative chemoradiotherapy prior to esophagectomy the treatment was not observed to be associated with major postoperative complications Citation[18]. In a study on the management of rectal cancer in the elderly it was concluded that age, taken as an independent variable, is not a contraindication to any specific type of therapy Citation[19]. Reviews on non-small lung cancer and aggressive non-Hodgkins lymphomas in the elderly have also been published Citation[20], Citation[21]. In anal cancer there are some previous studies on treatment of aged patients Citation[22], Citation[23] and the results with radiotherapy or chemoradiotherapy are found to be comparable to those achieved in younger age groups. The results of our study are in line with these earlier observations.
In our material the mean amount of permanent intercurrent diseases in patients over the age of 70 was only 1.05 (range 0–3). Thus these patients can be considered to have been in better general condition than patients of their age in average. The more frequent dose reductions of chemotherapy in patients with intercurrent diseases are probably the reason for the inferior local control figures in patients with comorbidities. The lower than normal incidence of intercurrent diseases is a logical explanation for the observed long life expectancy following successful cancer therapy. Elderly patients with no other potentially fatal diseases than cancer can be expected to have a longer than average life expectancy if the cancer can be cured, as other possible causes of death are fewer.
In summary, elderly patients with anal cancer should be treated similar to younger patients if there are no specific contraindications due to coexisting morbidities. Dose reductions in chemotherapy may be necessary in the aged patients in order to avoid excess acute toxicity. This must, however, be done with caution as it can lead to lower tumour control probability. The life expectancy in elderly patients after successful cancer therapy can be expected to be at least as long as in normal population.
References
- Life expectation tables: Statistics Finland. Media and Communication Unit. [email protected].
- Cancer Statistics: Finnish Cancer Registry. Personal communication.
- Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med 2000; 342: 792–800
- Clark MA, Hartley A, Geh JI. Cancer of the anal canal. Lancet Oncol 2004; 5: 149–57
- Nigro ND, Vaitkevicius VK, Considine B, Jr. Combined therapy for cancer of the anal canal: A preliminary report. Dis Colon Rectum 1974; 17: 354–6
- Arnott SJ, Cunningham D, Gallagher J, Gray R, Hardcastle J, Houghton J, et al. Epidermoid anal cancer: Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. Lancet 1996; 348: 1049–54
- Bosset JF, Pavy JJ, Roelofsen F, Bartelink H. Combined radiotherapy and chemotherapy for anal cancer. EORTC Radiotherapy and Gastrointestinal Cooperative Groups. Lancet 1997; 349: 205–6
- Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997; 15: 2040–9
- TNM classification of malignant tumors. Lippincott: UICC; 1997.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–6
- Balducci L, Beghe C. Cancer and age in the USA. Crit Rev Oncol Hematol 2001; 37: 137–45
- Carreca I, Balducci L, Extermann M. Cancer in the older person. Cancer Treat Rev 2005; 31: 380–402
- Vrakking AM, van der Heide A, van Delden JJ, Looman CW, Visser MH, van der Maas PJ. Medical decision-making for seriously ill non-elderly and elderly patients. Health Policy 2005; 75: 40–8
- Fried TR, Pollack DM, Tinetti ME. Factors associated with six-month mortality in recipients of community-based long-term care. J Am Geriatr Soc 1998; 46: 193–7
- Gosney MA. Clinical assessment of elderly people with cancer. Lancet Oncol 2005; 6: 790–7
- Bernardi D, Barzan L, Franchin G, Cinelli R, Balestreri L, Tirelli U, et al. Treatment of head and neck cancer in elderly patients: State of the art and guidelines. Crit Rev Oncol Hematol 2005; 53: 71–80
- Sarini J, Fournier C, Lefebvre JL, Bonafos G, Van JT, Coche-Dequeant B. Head and neck squamous cell carcinoma in elderly patients: A long-term retrospective review of 273 cases. Arch Otolaryngol Head Neck Surg 2001; 127: 1089–92
- Rice DC, Correa AM, Vaporciyan AA, Sodhi N, Smythe WR, Swisher SG, et al Preoperative chemoradiotherapy prior to esophagectomy in elderly patients is not associated with increased morbidity. Ann Thorac Surg 2005;79:391–7. Discussion 391–7.
- Abir F, Alva S, Longo WE. The management of rectal cancer in the elderly. Surg Oncol 2004; 13: 223–34
- Fratino L, Bernardi D. European experiences on aggressive non-Hodgkin's lymphoma in elderly patients. Crit Rev Oncol Hematol 2004; 51: 229–40
- Maione P, Perrone F, Gallo C, Manzione L, Piantedosi F, Barbera S, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: A prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol 2005; 23: 6865–72
- Allal AS, Obradovic M, Laurencet F, Roth AD, Spada A, Marti MC, et al. Treatment of anal carcinoma in the elderly: Feasibility and outcome of radical radiotherapy with or without concomitant chemotherapy. Cancer 1999; 85: 26–31
- de Gara CJ, Basrur V, Figueredo A, Goodyear M, Knight P. The influence of age on the management of anal cancer. Hepatogastroenterology 1995; 42: 73–6