Abstract
Determine feasibility and toxicity of preoperative short course pelvic CHART (25 Gy in 15 fractions over 5 days) for treatment of clinically resectable primary rectal tumours. Between 1998 and 2004, 20 patients with clinically staged T3 resectable rectal carcinoma were treated in this prospective pilot study with preoperative short course CHART to their pelvis. The aim was for total mesorectal excision within 7 days. Radiation toxicity, surgical morbidity, locoregional control (LRC), overall (OS), cause specific (CSS) and disease free survival (DFS) outcomes were documented. Nineteen of the 20 patients completed planned radiotherapy. One discontinued radiotherapy due to toxicity. All patients underwent potentially curative radical surgery. One patient developed grade 3, and three patients grade 2 gastrointestinal toxicity. With a median follow-up of 31 months (range 0.9–88), there is no grade 3, 4 or 5 late toxicity. Two patients experienced grade 2, and three patients grade 1 late bowel toxicity. Two patients died from postoperative complications, and two developed grade 2 abdominal wound infections. At 3 years LRC is 95% (95% CI 83–100), OS 72% (95% CI 51–94), CSS 86% (95% CI 68–100) and DFS 80% (95% CI 60–100). Two patients died from metastatic disease, one patient from a second primary and one patient is alive after successful resection of hepatic metastases. This small study suggests preoperative short course CHART for clinically resectable rectal carcinoma is feasible with acceptable compliance and tolerable side effects.
Since the Scandinavian data on short course preoperative radiotherapy (SCPRT) for the treatment of rectal carcinoma reported improvements in outcome, reduced local recurrence and longer overall survival Citation[1], Citation[2], the SCPRT regimen of 5×5 Gy fractions over one week, has become more popular among predominantly European centers. Short Potential Doubling Times (Tpot) of approximately 2–5 days, have been determined for human colorectal tumours Citation[3], and from a radiobiological perspective, short treatment times may have a better efficacy. Thus one of the main benefits of short/accelerated radiotherapy schedules is to reduce the repopulation of surviving tumour cells during treatment Citation[4]. However the high Biological Equivalent Dose (BED) calculation for hypofractionated regimens such as the Scandinavian SCPRT regimen raises concern regarding the potential for normal tissue toxicity Citation[5].
The acute toxicity associated with preoperative radiotherapy is generally transient, manageable and resolves within a few weeks. However, a more permanent lumbo-sacral plexopathy with persistent pain has been described with SCPRT Citation[6]. Other concerns include the increase in perineal wound infection after abdominoperineal resection in the postoperative period which can influence postoperative mortality and the late toxicity associated with large fraction sizes, accelerated treatment times and large field sizes. These effects have been noted with the use of large, Anterior/Posterior (APPA) fields in the Stockholm I Trial Citation[7]. However the Dutch total mesorectal excision (TME) trial and the Swedish rectal trial (SRT), comparing standard SCPRT using smaller multiple pelvic field techniques followed by surgery and surgery alone, also report some increase in toxicity. These include perineal wound infections, late faecal incontinence, blood and mucus loss, lower satisfaction with bowel function and increase in sexual dysfunction in the Dutch trial Citation[8–10]. At over 10 years follow-up of the SRT, although no overall increase in hospital admissions for complications, there is a trend to an increase in late admissions (>6 months after surgery) for gastrointestinal causes including bowel obstruction, nausea and abdominal pain and a trend to increase in fractures, not specifically related to the treatment field Citation[11]. Thus there remain concerns with some that 5×5 Gy is potentially too toxic and other groups have also designed different SCPRT regimens to address this issue. A recent paper from Austria describes a favourable outcome after 25 Gy in 10 fractions treating 2.5 Gy twice daily Citation[12].
We therefore proposed the investigation of a Continuous, Hyperfractionated, Accelerated Radiotherapy (CHART) regimen, 25 Gy in 15 fractions over 5 days, to achieve the same outcome with respect to tumour control but with less risk of late normal tissue toxicity when compared to standard short course radiotherapy as used in the Swedish and Dutch trials Citation[1], Citation[13].
CHART has been compared with conventionally fractionated radiotherapy in randomised phase III trials in the treatment of Head and Neck and non-small cell lung cancer (NSCLC) Citation[14], Citation[15]. In Head and Neck cancer CHART was proven to have the same local control but with reduced late toxicity compared to conventional radiotherapy Citation[14]. In NSCLC, Dische et al. reported a significant improvement in local control and overall survival Citation[15]. A previous pilot study of CHART (54 Gy in 36 fractions over 12 consecutive days) for the treatment of inoperable locally advanced or recurrent adenocarcinoma of the rectum was found to be feasible with regards toxicity and achieved rapid and sustained pain relief in their patient group Citation[16].
Using the linear quadratic (L-Q) isoeffect model (), 25 Gy in 5 fractions over 1 week, gives a BED of 37.5 Gy for tumour control using an alpha/beta ratio of 10 and 66.7 Gy for late normal tissue toxicity using an alpha/beta ratio of 3. For the CHART regimen, 25 Gy in 15 fractions over one week, the BED is lower at 29.2 Gy for tumour control and 39.0 Gy for late normal tissue toxicity, using the L-Q based isoeffect formula for multiple fractions per day () Citation[4]. Marsh et al. and, more recently Widder et al. have reported on SCPRT regimens with similar BED reductions for tumour control, 30Gy and 31.3 Gy respectively Citation[12], Citation[17]. The locoregional relapse associated with these trials, 12.8% after 96 months follow-up in the pre routine TME era and 2.1% after 4 years follow-up respectively, is similar to those reported from standard SCPRT regimens, 11% at 5 years for the SRT and 2.4% for the Dutch TME trial Citation[1], Citation[13]. Therefore we might expect similar tumour control but with less late normal tissue toxicity for the short course CHART regimen due to an improved therapeutic ratio.
Table I. Biological equivalent doses Citation[18].
The aim of this small non-randomised pilot study for clinically resectable rectal cancer was to assess the feasibility and toxicity of a preoperative short-course CHART regimen (25 Gy in 15 fractions over 5 days).
Methods and materials
Twenty patients with clinically staged resectable T3, histologically confirmed rectal adenocarcinoma from two cancer units were prospectively entered into this pilot study between 1998 and 2004. This is a consecutive series of patients from two clinical oncologists’ practices entered during the time period. During the same period patients with unresectable or borderline resectable cancers were treated with neoadjuvant chemoradiation with the aim of downstaging, and only a small proportion of resectable T3 cases received the Scandinavian SCPRT regimen of 5×5 Gy over one week.
Preoperative staging assessment included digital rectal examination, sigmoidoscopy and CT-scan. Magnetic Resonance Imaging was not routine with only one patient having an MRI in this series. These patients were selected for short course preoperative radiotherapy based on having T3 disease with or without nodal involvement or due to having low rectal disease that required abdominoperineal resection and where the margins of resection were expected to be very close. Patients were ineligible if they had metastases on CT staging investigations. All patients underwent TME surgery.
Acute toxicity was recorded prospectively weekly during radiotherapy, and thereafter up to and including the week of surgery. Surgical morbidity and complications were also documented up to 30 days following surgery.
Although only small numbers, follow-up data has been collected on loco-regional and distant recurrence, and evidence of late morbidity documented on a trial proforma prospectively via 6 monthly clinic visits or telephone interview. Timing of subsequent events and follow-up was measured from the first day of radiotherapy.
Radiotherapy was planned with the patient in the prone position with a full bladder. Oral gastrograffin and rectal barium were given to help delineate the bowel. A 3 field plan or a 4 field box technique was employed. Treatment target volume aimed to encompass potential extension of the tumour into the peri-rectal nodes lying beneath S3, the mesorectum and the posterior pelvis. Inclusion of the external iliac lymph nodes occurred if structures such as the prostate, bladder or vagina were thought to be involved. The prescribed dose was specified at the intersection of the central axis of the beams. Isodose distributions in three transverse sections, central and two off-axes, were generated for dosimetric considerations and quality control. The treatment was delivered 3 times per day with a 6 hour break between fractions (0800 h, 1400 h, and 2000 h), delivering 1.67 Gy per fraction. Treatment was delivered continuously for 5 days and all fields were treated at each fraction of radiotherapy.
The major endpoints were acute toxicity and late toxicity which was scored using the RTOG (Radiation Therapy and Oncology Group)/EORTC (European Organisation for Research and Treatment of Cancer) ‘Acute and Late Radiation Morbidity Scoring Criteria’ Citation[18]; local recurrence rate; overall, cause specific and disease free survival.
Loco-regional control, overall, cause specific and disease free survival were assessed using the Kaplan-Meier method and were measured from the date of starting radiotherapy to the date of event, last follow-up or death (those patients who died from intercurrent disease were censored at the time of death).
Ethical approval was obtained prior to initiation of the trial. Written patient information sheets were supplied and informed consent was obtained from each patient by the treating physician prior to entry to the trial.
Results
Compliance
Of the 20 patients enrolled, all started radiotherapy and 19 completed the full planned radiotherapy. One patient received 16.72 Gy, stopping after 10 fractions due to development of grade 3 nausea and vomiting. This patient recovered from the acute toxicity and proceeded to surgery according to protocol, but subsequently died of metastatic disease 8 months later.
Timing of surgery
Nineteen patients underwent immediate surgery as intended in the protocol. The median time interval between the start of radiotherapy and surgery was 9.0 days with a range of 8–29 days (8, 8, 8, 8, 8, 8, 8, 8, 9, 9, 9, 9, 9, 10, 11, 14, 16, 16, 29 days). One patient was deemed to be unresectable at laparotomy, and thus received 5 Fluorouracil (5FU) based chemotherapy with a view to further downsizing. This patient was successfully resected after a 6 month delay.
Histopathological data
There was a high proportion (35%) of pathological T1/T2N0 disease in this series. All circumferential resection margins (CRMs) reported were > 1 mm from the periphery of the tumour and thus considered uninvolved. The CRM ranged from 2 to 20 mm ().
Table II. Patient and tumour characteristics.
Locoregional control and survival
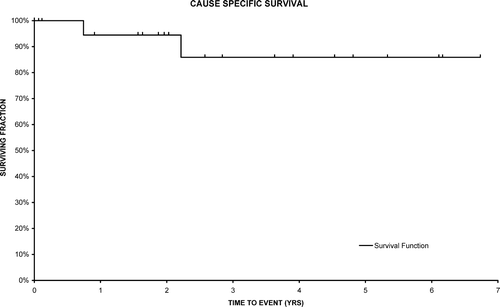
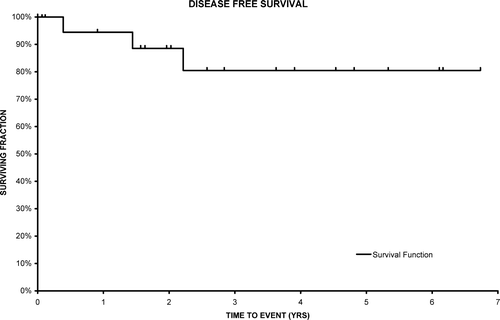
The actuarial locoregional control rate is 95% at 3 years (95% CI 83–100). The overall survival is 72% (95% CI 51–94) and cause specific survival related to adenocarcinoma of the rectum is 86% (95% CI 68–100) (). Disease free survival is 80% at 3 years (95% CI 60–100) () and there was one regional recurrence.
Acute toxicity
One patient experienced grade 3 upper gastrointestinal (GI) toxicity requiring hospitalisation and 24 hours of intravenous rehydration during their radiotherapy, and three patients experienced grade 2 gastrointestinal (GI) toxicity. There was no serious haematological toxicity. There was no grade 4 acute toxicity reported. Three patients experienced grade 2 acute skin toxicity, and subsequently developed abdominal wound infections in the postoperative period that responded to antibiotics and did not prolong hospital stay. There were no perineal wound problems noted; one patient experienced grade 1 skin toxicity; one grade 1 upper and four grade 1 lower GI toxicity was reported. ()
Table III. Acute toxicity scoring.
Surgical complications and morbidity
Two patients died in the postoperative period. One patient developed multi-organ failure associated with Methicillin Resistant Staphylococus Aureus (MRSA) septicaemia, originally cultured from urine. The other postoperative death was associated with an initial postoperative intra-abdominal bleed. This patient underwent further surgery within the first 24 hours achieving haemostasis but was unable to be weaned from the ventilator and went on to develop pneumonia, associated septicaemia and multi-organ failure.
Late toxicity
With a median follow-up of 31 months (range 0.9–88), two patients developed grade 2 late bowel toxicity and three patients experienced grade 1 bowel toxicity. There was no grade 3, 4 or 5 late toxicity. There was no late urinary, skin, subcutaneous or neurological effect reported. Other complications noted but not included in the RTOG/EORTC scoring system include three uncomplicated, incisional hernias and two patients report impotence. Both patients underwent abdominoperineal resection of their rectal carcinomas. ()
Table IV. Late toxicity scoring.
Discussion
This small pilot study reports acceptable survival rates with 3 year overall survival of 72%, cause specific survival of 86% and disease free survival 80%. There was only one loco-regional failure in the patient that did not complete their planned radiotherapy. The confidence intervals are noted to be broad. These outcomes are however encouraging particularly in view of the high proportion of low rectal tumours included in this series (50%), which tend to have poorer outcomes compared to mid and upper rectal sites.
The acute toxicity associated with our series is similar to that reported previously with predominant grade 1 acute bowel toxicity. Perioperatively there was no perineal wound infection but there were two grade 1 abdominal wound infections. Of more concern is a high level of postoperative mortality of 10% when the mortality in other SCPRT studies is around 2–4% in the radiotherapy groups Citation[1], Citation[2], Citation[13]. It is impossible to draw firm conclusions as to whether this outcome reflects a real effect of the radiotherapy as this is a small series. The Dutch trial does report that if time to surgery was greater than 8 days, then the 180 day postoperative mortality was significantly higher, 8.4% v 4.1%, p = 0.02. They note mortality was observed mainly in older patients, over 75 years of age Citation[19]. Over half of the patients in this series waited > 8 days which may have contributed to the increase in postoperative mortality seen and the two postoperative deaths were of patients aged 78 and 79 years which is consistent with the Dutch results of increasing mortality with an increase in age. However again, because of small numbers, it is difficult to make these conclusions. One of our patients died with probable pelvic sepsis. There is no confirmed evidence of an increase in pelvic sepsis with SCPRT and thus we cannot assume that this event was a result of their radiotherapy. We believe therefore, that this mortality rate may be more a reflection of the small numbers in the series rather than an effect of the radiotherapy. With respect to the late toxicity, we acknowledge that the follow-up is short. At 6.7 years there is no grade 3, 4 or 5 toxicity reported with a low percentage of grade 1 and 2 gastrointestinal toxicity. Thus, the preliminary evidence from this series suggests a low level of concern about late effects with this short course CHART regimen.
There is a significant number of pathological pT1/T2N0 tumours in this series (30%). There may be a tendency to overstage rectal cancer preoperatively. In the randomised studies of clinically staged T3/T4 rectal cancer, the percentage of pT1T2N0 in the control or postoperative arm was 18% in the German study Citation[20], 28% in the Dutch study Citation[13] and 39% in the Polish study Citation[21] respectively. This overstaging may contribute to the favourable survival and local control outcomes reported.
In the present study the high proportion of low stage disease may also be partly associated with the unit policy to offer preoperative treatment to those with low rectal tumours regardless of TNM stage, based on the concern regarding difficulty in achieving a negative CRM in this region. However, it is noted that there are no reported positive CRM in this series of patients. Data from the Netherlands addressing the issue of positive CRM, by Marijnen et al., comparing TME with or without preoperative short course radiotherapy reported a positive CRM rate of 17.2% Citation[22]. The lack of positive CRM in this series may be a reflection of the small sample size in this trial or because of initial clinical over-staging particularly as MRI was not routinely used preoperatively. It has been recognised that a degree of inaccuracy in clinical staging can occur comparing the reporting of preoperative radiological imaging to pathological staging Citation[23]. Another possible explanation is that an element of down-staging occurred. Further support for this theory comes from the data by Graf et al. looking at the downstaging effect on 1316 preoperative short course specimens Citation[24]. They found a reduction in size of the primary tumours and reduced number of lymph nodes involved if the interval from the start of radiotherapy to surgery was 10 days or greater. Although median interval to surgery was generally short in this trial at 9.0 days, six patients’ time to surgery was greater and they may therefore have experienced a downstaging effect.
Preoperative adjuvant radiotherapy with or without synchronous chemotherapy has also been shown to improve loco-regional control in a number of randomised trials over surgery alone or postoperative chemoradiation Citation[1], Citation[17], Citation[20]. The Swedish Trial is the only adjuvant radiotherapy trial to report an improvement in overall survival ie 58% v 48% at 5 years, p = 0.004 Citation[1]. Two meta-analyses of adjuvant radiotherapy trials, Camma et al. Citation[25] and The Colorectal Cancer Collaborative Group Citation[26] support these findings, although the impact on survival is less clear. Short course preoperative radiotherapy has also been shown to improve local relapse rates even when TME surgery is performed as reported by the Dutch Colorectal Group (2.4% v 8.2% at 2 years, p < 0.001) Citation[13]. These results have led to the popular use of this regimen in Europe and particularly the UK.
The Medical Research Council (MRC) CR07 trial which compares SCPRT, 25 Gy in 5 fractions over 1 week, to selective postoperative radiotherapy, 45 Gy in 25 fractions over 5 weeks with concurrent chemotherapy for those patients with R1 resection has now completed accrual. The recently reported preliminary results confirm that SCPRT confers a significant reduction in local recurrence and improves 3 year DFS Citation[27]. The published results should generate hypotheses in combination with the Dutch TME trial as to which patients most benefit from the 5 fraction technique preoperatively. The role of a short course preoperative CHART regimen, 25 Gy in 15 fractions over 5 days, for resectable rectal cancer may then be better defined. In addition, recent evidence from large randomised trials support the integration of 5FU into radiation schedules Citation[20], Citation[28], Citation[29]. It may be possible to integrate concurrent chemotherapy with this CHART regimen because of the small size of the individual fractions and associated favourable therapeutic ratio of BED outcomes. This strategy could further improve outcomes of survival and loco-regional control.
Conclusion
This schedule of short course CHART as preoperative therapy for clinically resectable rectal cancer followed by TME is feasible, and appears associated with low acute and late toxicity. However, two postoperative deaths should raise a note of caution even though these are not clearly associated with the regimen. Local control is encouraging and therefore warrants further evaluation of this regimen. Incorporation of concurrent chemotherapy could also be considered an option for investigation in the future.
References
- Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 1997; 336: 980–7
- Martling A, Holm T, Johansson H, Rutqvist LE, Cedermark B, Stockholm Cancer Study Group. The Stockholm II Trial on preoperative radiotherapy in rectal carcinoma: Long-term follow-up of a population-based study. Cancer 2001; 92: 896–902
- Rew DA, Wilson GD, Taylor I, Weaver PC. Proliferation characteristics of human colorectal carcinomas measured in vivo. Br J Surg 1991; 78: 60–6
- Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumour clonogen repopulation during radiotherapy. Acta Oncol 1988; 27: 131–46
- Steel G. ( editor). Basic Clinical Radiobiology. 3rd edition. London: Edward Arnold Publishers; 2002.
- Frykholm JG, Sintorn K, Montelius A, Jung B, Pahlman L, Glimelius B. Acute lumbosacral plexopathy after preoperative radiotherapy in rectal carcinoma. Radiother Oncol 1996; 38: 121–30
- Cedermark B, Johansson H, Rutqvist LE, Wilking N. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma. A prospective randomized trial. Stockholm Colorectal Cancer Study Group. Cancer 1995; 75: 2269–75
- Marijnen CAM, Kapiteijn E, van de Velde CJ, Martijn H, Steup WH, Wiggers T, et al. Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: Report of a Multicenter randomized trial. J Clin Oncol 2002; 20: 817–25
- Peeters KC, van de Velde CJ, Leer JW, Martijn H, Juggeburt JM, Kranenberg EK, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients-a Dutch colorectal cancer group study. J Clin Oncol 2005; 23: 6199–206
- Marijnen CAM, vande Velde CJ, Putter H, van den Brink M, Maas CP, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: Report of a multicenter randomized trial. J Clin Oncol 2005; 23: 1847–58
- Birgisson H, Pahlman L, Gunnarsson U, Glimelius B, Swedish Rectal Cancer Tria Group. Adverse effects of preoperative radiation therapy for rectal cancer: Long-term follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol 2005; 23: 8697–705
- Widder J, Herbst F, Dobrowsky W, Schmid R, Pokrajac B, Jech B, et al. Preoperative short-term radiation therapy (25Gy, 2.5Gy twice daily) for primary resectable rectal cancer (phase II). Br J Cancer 2005; 92: 1209–14
- Kapiteeijn E, Marijnen C, Nagtegaal I. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001; 345: 638–46
- Saunders M, Dische S, Barrett A, Harvey A, Griffiths G, Palmar M. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: Mature data from the randomised multicentre trial. CHART Steering committee. Radiother Oncol 1999; 52: 137–48
- Dische S, Saunders M, Barrett A, Harvey A, Gibson D, Parmar M, et al. A randomised multicentre trial of CHART versus conventional radiotherapy in head and neck cancer. Radiother Oncol 1997; 44: 123–36
- Glynne-Jones R, Saunders M.I, Hoskin P, et al. A pilot of continuous, hyperfractionated, accelerated radiotherapy in rectal adenocarcinoma. Clin Oncol 1999; 11: 334–9
- Marsh PJ, James RD, Schofield PF. Adjuvant preoperative radiotherapy for locally advanced rectal carcinoma. Results of a prospective, randomized trial. Dis Colon Rectum 1994; 37: 1205–14
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–6
- Marijnen CAM, Leer JWH, Putter H, Kapiteijn E, van Krieken JH, Noordijk EM, et al. Interval between preoperative radiotherapy and surgery influences postoperative mortality in rectal cancer patients: The sooner the better. Eur J Cancer 2001; 37: S273
- Sauer R, Becker H, Hohenber W, Rodel C, Witteking C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–40
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebneck M, Pudelko M, et al. Sphincter preservation following preoperative radiotherapy for rectal carcinoma: Report of a randomised trial comparing short-term radiotherapy vs conventionally fractionated radiochemotherapy. Radiother Oncol 2004; 72: 15–24
- Marijnen CAM, Nagtegaal ID, Kapiteijn E, Kranenbarg EK, Noordijk EM, van Krieken JH, et al. Radiotherapy does not compensate for positive resection margins in rectal cancer patients: Report of a multicenter randomised trial. Int J Radiat Oncol Biol Phys 2003;55:1311–20.
- Mathur P, Smith JJ, Ramsey C, Owen M, Thorpe A, Karim S, et al. Comparison of CT and MRI in the pre-operative staging of rectal adenocarcinoma and prediction of circumferential resection margin involvement by MRI. Colorectal Dis 2003; 5: 396–401
- Graf W, Dahlberg M, Osman MM, Holmberg L, Pahlman L, Glimelius B, et al. Short-term preoperative radiotherapy results in down-staging of rectal cancer: A study of 1316 patients. Radiother Oncol 1997; 43: 133–7
- Camma C, Giunta M, Fiorica F, Pagliaro L, Craxi A, Cottone M. Preoperative radiotherapy for resectable rectal rancer: A metanalysis. JAMA. 2000; 284: 100
- Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: A systemic overview of 8507 patients from 22 randomised trials. Lancet 2001; 358: 1291–304
- Sebag-Montefiore D, Steele R, Quirke P, Grieve B, Khama S, Monson J, et al. Routine short course pre-op radiotherapy or selective post-op chemoradiotherapy for resectable rectal cancer? Preliminary results of the MRC CR07 randomised trial. J Clin Oncol PROC ASCO 2006;24: S18,S148, Abstract 3511.
- Bosset JF, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, et al Preoperative radiation (Preop RT) in rectal cancer: Effect and timing of additional chemotherapy (CT) 5-year results of the EORTC 22921 trial. Proc Am Soc Clin Oncol 2005; Abstract No 3505.
- Gerard J, Bonnetain F, Conroy T, Chapet, O, Bouche O, Closon-Dejardin MT, et al Preoperative (preop) radiotherapy (RT) + 5 FU/folinic acid (FA) in T3-4 rectal cancers: Results of the FFCD 9203 randomized trial. Proc Am Soc Clin Oncol 2005; Abstract No 3504.