Abstract
The feasibility, toxicity and tumor response of stereotactic body radiation therapy (SBRT) for treatment of primary and metastastic liver tumors was investigated. From October 2002 until June 2006, 25 patients not suitable for other local treatments were entered in the study. In total 45 lesions were treated, 34 metastases and 11 hepatocellular carcinoma (HCC). Median follow-up was 12.9 months (range 0.5–31). Median lesion size was 3.2 cm (range 0.5–7.2) and median volume 22.2 cm3 (range 1.1–322). Patients with metastases, HCC without cirrhosis, and HCC < 4 cm with cirrhosis were mostly treated with 3×12.5 Gy. Patients with HCC ≥4cm and cirrhosis received 5×5 Gy or 3×10 Gy. The prescription isodose was 65%. Acute toxicity was scored following the Common Toxicity Criteria and late toxicity with the SOMA/LENT classification. Local failures were observed in two HCC and two metastases. Local control rates at 1 and 2 years for the whole group were 94% and 82%. Acute toxicity grade ≥3 was seen in four patients; one HCC patient with Child B developed a liver failure together with an infection and died (grade 5), two metastases patients presented elevation of gamma glutamyl transferase (grade 3) and another asthenia (grade 3). Late toxicity was observed in one metastases patient who developed a portal hypertension syndrome with melena (grade 3). SBRT was feasible, with acceptable toxicity and encouraging local control. Optimal dose-fractionation schemes for HCC with cirrhosis have to be found. Extreme caution should be used for patients with Child B because of a high toxicity risk.
Hepatocellular carcinoma (HCC) and colorectal cancer are among the five most common causes of cancer mortality in the world Citation[1]. As many as 50–70% of patients diagnosed of colorectal cancer will present liver involvement during follow-up, being the only site of recurrence in half of these patients Citation[2]. Surgery is accepted as a potentially curative option with survival rates at 5 years of 50–70% for early diagnosed HCC, and 25–35% for liver metastases when disease is confined to the liver Citation[2–5]. However, the majority of patients are not eligible for surgery because of liver function impairment, diminished liver function capacity after several resections, location of the lesion in centrally located segments or concomitant medical diseases Citation[4–6]. For patients who are not suitable for surgery, other local treatment methods, especially radiofrequency ablation (RFA) are emerging as alternative curative options but, the proximity of the lesion to the gall bladder or main vessels, the subdiaphragmatic location, or the presence of a non-echogenic lesion (for ultrasound-guided RFA) constitute major problems to apply this treatment Citation[7]. Radiotherapy, alone or in combination with transarterial chemoembolization has become a potential new treatment option for primary and metastatic liver tumors around the world Citation[8–10]. Stereotactic body radiation therapy (SBRT) has no strict restrictions regarding lesion location, and offers the possibility of a high precision non-invasive treatment, using small margins Citation[11]. The aim of this paper was to assess feasibility, toxicity and tumor response of SBRT as a new local treatment modality for primary and metastatic liver tumors in our patient population.
Materials and methods
Patient characteristics
Patients included were those with primary or metastatic tumors confined to the liver, and not eligible for surgery or other local treatment (RFA). The Karnofsky index was at least 80%. The Child-Pugh grade for HCC patients was A-B. With the maximum lesion size allowed being 7 cm, a maximum of three lesions was acceptable for the protocol. and summarize the patient characteristics of this study. From October 2002 until June 2006, eight patients were treated for 11 primary liver tumors (HCC) and 17 patients for 34 metastases. Median age was 63 years (range 37–81). Gender distribution was five females, 20 males. Median tumor size was 3.2 cm (range 0.5–7.2 cm) and median tumor volume 22.2 cm3 (range 1.1–322). All patients with primary tumors, except one, had cirrhotic livers. In contrast, in the metastases group, only one patient had liver function impairment with signs of portal hypertension (cardial and esophageal varices). Probably, this was due to portal vein thrombosis developed after previous radiotherapy performed elsewhere because of other liver metastases.
Table I. HCC. Patient characteristics.
Table II. Liver metastases. Patient characteristics.
Dose-fractionation schemes
The dose was prescribed at the 65% isodose that surrounded the PTV. Patients with liver metastases, HCC without cirrhosis, and HCC < 4 cm and cirrhosis were mostly treated with 3 fractions of 12.5 Gy. Three patients with liver metastases have been treated with 3 fractions of 10 Gy. One patient because of the presence of only microscopic disease (after non-radical microscopic surgery), another patient because of the small bowel in the high dose area, and the third one because of the amount of normal liver involved in the high dose region. For patients with HCC ≥4 cm and cirrhosis, treatment consisted initially, for the first three patients, on 5 fractions of 5 Gy (5×5 Gy). Because no grade 3–4 toxicity was observed but a local failure was evidenced in two of them very close after treatment (see below), retreatment was performed using 3 fractions of 8 Gy without evidence of severe toxicity. These two patients were considered as a failure for the actuarial local control calculations. The dose was increased for the last patient to 3 fractions of 10 Gy. Treatment fractions were delivered every second day. Overall treatment time was 5–6 days for 3 fractions and 10 days for 5 fractions.
Treatment preparation and execution
For SBRT patients were positioned in the Elekta Stereotactic Body Frame (SBF) (Elekta Oncology Systems, Stockholm, Sweden) with maximum tolerable abdominal compression to reduce respiratory tumor motion. All patients had a planning, an arterial and a venous contrast CT-scan. The rim of contrast enhancement was taken as boundary of the clinical target volume (CTV) and was delineated on the arterial and venous contrast CT-scans and summed to construct the definitive 3-D CTV. The tumor delineations were reviewed by an experienced radiologist (S. M. Hussain/ S. Dwarkasing). Initially, the applied PTV margin was based on the Karolinska experience (5–10 mm) Citation[12]. Currently, implanted gold fiducials and fluoroscopy are used to assess residual tumor motion in all directions and margins are individualized.
Treatment plans (A and B) were generated with the Cadplan treatment planning system (Varian Oncology Systems, Palo Alto, CA) with 4–10 coplanar and non-coplanar beams. The following normal tissue constraints were used Citation[13]: for normal liver D33%<21 Gy, D50%<15 Gy, for bowel, duodenum, stomach and esophagus D5cc<21 Gy, for spinal cord Dmax<15 Gy and for kidney D33%<15 Gy.
Figure 1A. T2-weighted MR image shows one hyperintense metastasis in segment 7 with a small satellite lesion (arrow).
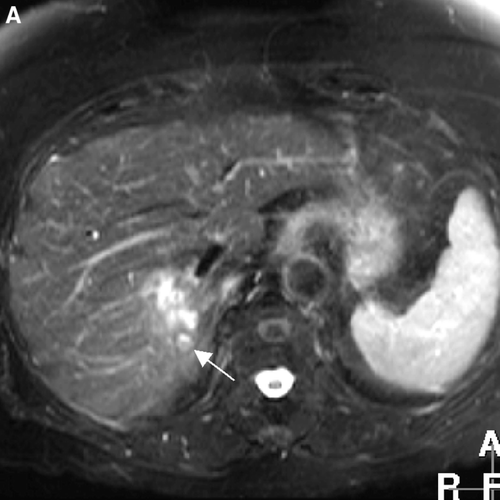
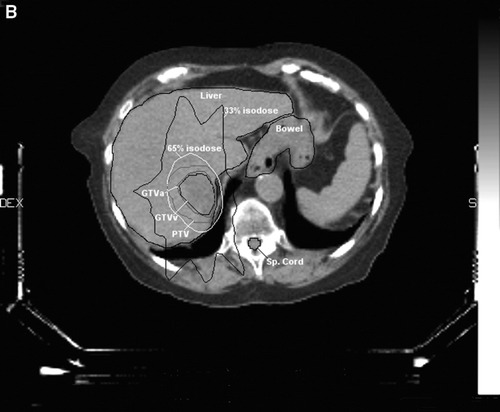
Figure 1B. CT-planning showing the delineations corresponding to the two liver metastases in segment 7, both included in one target, the GTV arterial and venous phase, the organs at risk (liver, bowel and spinal cord), the PTV surrounded by the 65% isodose and the 33% isodose.
The treatments were delivered with a Siemens Primus linear accelerator (Siemens Oncology Systems, Concord, CA). Just prior to each treatment fraction a contrast CT scan was acquired to assess tumor motion and bony anatomy displacements in the SBF. Also, electronic portal images were used to exclude movements of the patient (bony anatomy) in the SBF during transport from the CT-scanner to the linear accelerator and to verify applied SBF set-up corrections, in case of detected tumor displacements in the SBF at the CT-scanner.
Follow-up and definition of local failure
All patients had a multiphase gadolinium enhanced liver MRI scan, a liver function test, and tumor marker assessment between 4 and 6 weeks prior to treatment planning, and at 1, 2, 3 months after treatment and periodically every 3 months during the first 2 years, and every 6 months thereafter. The tumor size was evaluated on a MRI axial reconstruction and the volumes with the contrast CT-scan delineations. Local failure was defined as increase in tumor size and/or steady increase of tumor marker values above normal, without evidence of new intra- or extrahepatic lesions at any point during the follow-up. Median follow up for local control was 12.9 months (range 0.5–31).
Toxicity evaluation
Acute toxicity was evaluated during the first 3 months after treatment with the Common Toxicity Criteria (CTC), version 2.0 of the National Cancer Institute. The SOMA/ LENT grading system was used to score the late toxicity. Radiation induced liver disease (RILD) was defined as, anicteric ascites and elevation of alkaline phosphatase levels to at least two fold increase above the pretreatment values in absence of tumor progression, classic Citation[14], or hepatic toxicity grade 3 or higher according to the CTC also in absence of tumor progression, non-classic Citation[15], Citation[16].
Statistics
To assess local control and survival, Kaplan-Meier curves were generated with SPSS, version 11.5.
This retrospectively analyzed phase I-II study was approved by the Ethical Commission of Erasmus MC and all patients have given their written consent.
Results
Local control
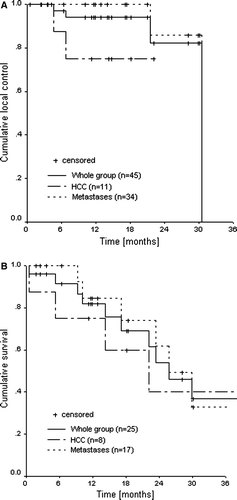
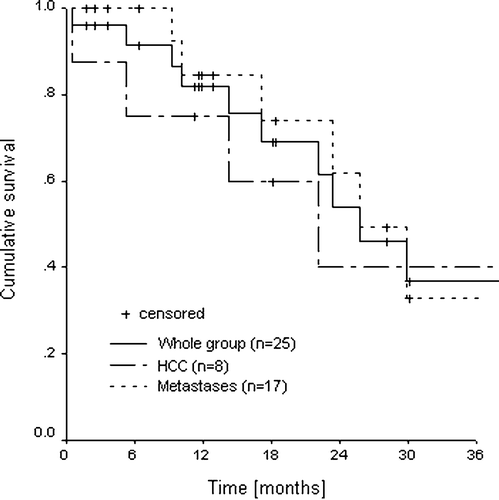
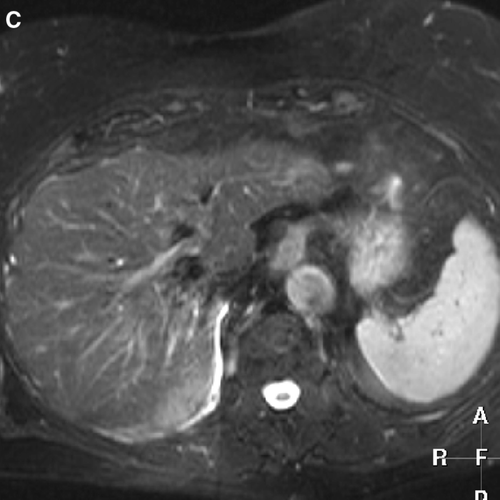
Local failure was observed in four out of 45 lesions in four patients (2 HCC, 2 metastases). One HCC patient presented a steady increase of AFP 7 months after treatment without increase in tumor size but with an active rest lesion on a PET scan. The other HCC patient showed an in field regrowth after initial decrease of lesion size and elevated AFP 4 months post-treatment. These two patients were treated with 5×5 Gy. Two metastases patients presented an in field regrowth after an initial complete remission with an increase of the CEA level at 31 and 21 months after treatment. Both patients were treated with 3×12.5 Gy. An example to illustrate a complete remission is shown in C. The actuarial one and two year local control rates were 94% and 82% for the whole group and 100% and 86% for the metastases group, respectively (). For the HCC the one year and twenty-two months local control probability were 75% (maximum follow-up of a HCC in local control is 22 months). Crude local control rates for liver metastases and HCC were 94% and 82%, respectively. Actuarial overall survival rates at one and two years () were 82% and 54% for the whole group; 85% and 62% for the metastases group and 75% and 40% for HCC patients.
Figure 1C. T2-weighted MR image shows complete remission with morphological parenchymal changes due to radiation hepatitis 21 months after treatment.
Toxicity
Changes in the liver function parameters, grade 1–2, were present in all the patients except one (grade 0) in the first 3 months after treatment. Within the HCC group, one episode of RILD classic and non-classic was observed. Two weeks after treatment, a Child B patient presented hepatic toxicity grade 4 with signs of decompensated portal hypertension, bleeding from esophageal varices, and fever from a urinary infection. The patient died within the first month after treatment and the toxicity was evaluated as grade 5. Two patients presented ascites grade 2 with less than two fold increased alkaline phosphatase and hepatic toxicity (CTC) less than grade 3, responding well to temporary diuretic medication. No late toxicity was found. In the metastases group, two episodes of RILD non-classic were found in the first 3 months after treatment. Two patients presented an elevation of gamma glutamyl transpherase (GGT) grade 3. In one case the increase was isolated, without any symptom or parallel increase of other liver function parameters. In the other, an increase of the other liver parameters and asthenia (both grade 2) was observed. One patient developed asthenia grade 3 with hepatic toxicity grade 2 during the first month after treatment and recovered spontaneously during the second month. Also, one episode of ascites grade 2 with less than two times increase of alkaline phosphatase was observed in one patient after being treated three times with radiotherapy (last two in our center) and previously with surgery. Late toxicity could also be present in this patient because of the development of a portal hypertension syndrome with one melena episode that was evaluated as SOMA grade 3. No esophagus, stomach, bowel or kidney toxicity was found.
Discussion
Local control whole group
Our results, with local control rates of 94% and 82% at 1 and 2 years, respectively, are in accordance with those published from similar studies in the literature () Citation[9], Citation[13], Citation[17–20]. The better local control compared to Wulf et al. Citation[13] and Shefter et al. Citation[19] can perhaps be explained by the higher doses used in the present study. Herfarth et al., in a recent publication Citation[21], have reported results of 70 patients, 35 of the first phase I/II trial Citation[18] (4 primary tumors, 51 metastases) and 35 treated after the trial was closed (51 metastases, mainly colorectal). Actuarial local control rates were 66% at 18 months and 60% at 2 years. The lower results in comparison with those from the first 35 patients were due to a poor local control rate of the colorectal metastases group (see metastases section).
Table III. Comparison of local control and survival between studies using hypofractionated radiotherapy.
Separate comparisons for HCC and metastases are difficult to perform, as most studies don't make this separation in their analysis.
Local control HCC
Blomgren et al. Citation[9] reported a crude local control at last follow-up (median 12 months) of 100%. Our result of 80% crude local control at one year is lower but this needs further consideration. All tumors in the present study treated with 3×12.5 Gy stayed in local control. The difference with the results from Blomgren et al. is due to our poor local control achieved for large tumors with cirrhosis, probably because of the too low total dose delivered (5×5 Gy). A clear dose effect relationship for HCC has been established in the literature by Dawson et al. and Park et al. Citation[10], Citation[22]; eventually the combination with a large size, as demonstrated by Wada et al. Citation[20] could have influenced the low local control rate.
Local control metastases
Kavanagh et al. observed local control rates of 100% and 93% at 1 and 2 years, in 36 liver metastases patients treated with 3 fractions of 20 Gy (pers. comm.). Probably, the delivered higher doses have decreased the local failure rate in comparison with our study. Herfarth et al. Citation[21] performed a separate analysis considering only the metastases patients. They observed that colorectal metastases presented a poorer local control than those with other histology (45% vs 91% after 18 months), especially for those treated previously with systemic chemotherapy, that could have selected radioresistant cells. We observed better local control rates even with a population including mainly colorectal metastases (27 of 34 lesions). It might be possible that our patients were treated less with chemotherapy and this could explain the observed difference.
Toxicity whole group
Comparison with other groups is difficult because of different or not mentioned scoring systems used to report toxicity. However, the data from the literature Citation[9], Citation[13], Citation[18–20] seem to suggest that published toxicity for the whole group is lower than in our study.
Toxicity HCC
Cheng et al. and Jiang et al. Citation[15], Citation[16] have demonstrated that patients with cirrhosis Child-Pugh grade B have a high risk to develop toxicity (RILD). As well, Cheng et al. conclude that hepatitis B virus (HBV) was also associated with higher susceptibility for RILD but the mean liver dose and the V30Gy (percentage of normal liver volume that received a radiation dose >30 Gy) were not statistical significantly correlated. This is in agreement with our experience. We had two patients with Child-Pugh B that also carried HBV. One who developed tumor progression and could therefore not be evaluated for toxicity. The other patient presented fever two weeks after treatment, due to an infection, with signs of increased portal hypertension and RILD, progressing to liver failure and death. Although it is known that in cirrhotic patients a liver decompensation can be associated with infections, possibly the radiation induced edema increased the portal pressure and the subsequent bleeding contributed to deteriorate the unstable liver function. This patient had a V30Gy of 6% and the mean and the median liver dose were also low, 8.6 and 3.4 Gy (converted to 2 Gy per fraction with α/β = 2). HCC with cirrhosis Child-Pugh A and patients without cirrhosis, regardless of size, didn't show any toxicity grade ≥3. Possibly, the absence of severe liver function impairment makes them less susceptible to develop complications.
Toxicity metastases
Within the metastases group, we observed grade 3 hepatic toxicity in two patients based on an increased GGT. The published phase I trial from the Colorado and Indiana groups Citation[23] showed that for metastases, escalating the dose until 60 Gy delivered in 3 fractions was possible, without reaching the maximum tolerated dose for grade 3–4 toxicity. In this study, at least 35% of normal liver, or 700 ml, estimating a normal liver volume of 2 000 ml, had to receive a total dose less than 15 Gy. Analysis of the dose-volume histogram of the two patients with hepatic toxicity showed that 53% and 60% of the normal liver received 15 Gy or less corresponding to 638 and 639 ml, respectively. The first patient was treated because of two targets and the second one had a small liver volume after previous operations. The patient with asthenia grade 3 was treated with chemotherapy and resection prior to radiotherapy what could have influenced the development of more constitutional symptoms.
Conclusions
SBRT was feasible, with an acceptable toxicity and encouraging local control, especially for liver metastases. More studies including larger numbers of patients are necessary to verify these results and to find optimal dose-fractionation schemes for HCC patients with cirrhotic livers. If patients with Child-Pugh B are considered for treatment extreme caution should be used because of the high risk to develop toxicity.
The authors would like to thank P. T. N. Pattynama and S. Dwarkasing for their valuable contributions. The authors don't have conflicts of interest to declare.
References
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003; 362(9399)1907–17
- Poston GJ. Surgical strategies for colorectal liver metastases. Surg Oncol 2004; 13: 125–36
- Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol 2005; 40: 225–35
- Verhoef C, Visser O, de Man RA, de Wilt JH, IJzermans JN, Janssen ML. Hepatocellular carcinoma in the Netherlands incidence, treatment and survival patterns. Eur J Cancer 2004; 40: 1530–8
- Yoon SS, Tanabe KK. Surgical treatment and other regional treatments for colorectal cancer liver metastases. Oncologist 1999; 4: 197–208
- Llovet JM, Beaugrand M. Hepatocellular carcinoma: Present status and future prospects. J Hepatol 2003; 38(Suppl 1)S136–S149
- Decadt B, Siriwardena AK. Radiofrequency ablation of liver tumours: Systematic review. Lancet Oncol 2004; 5: 550–60
- Blomgren H, Lax I, Naslund I, Svanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995; 34: 861–70
- Blomgren H, Lax I, Göranson H, Kraepelien T. Radiosurgery for tumors in the body: Clinical experience using a new method. J Radiosurgery 1998; 1: 63–74
- Dawson LA, McGinn CJ, Normolle D, Ten Haken RK, Walker S, Ensminger W, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol 2000; 18: 2210–8
- Lax I, Blomgren H, Larson D, Naslund I. Extracranial stereotactic radiosurgery of localized targets. J Radiosurgery 1998; 1: 135–48
- Lax I, Blomgren H, Naslund I, Svanstrom R. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol 1994; 33: 677–83
- Wulf J, Hadinger U, Oppitz U, Thiele W, Ness-Dourdoumas R, Flentje M. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther Onkol 2001; 177: 645–55
- Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002; 53: 810–21
- Cheng JC, Wu JK, Lee PC, Liu HS, Jian JJ, Lin YM, et al. Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. Int J Radiat Oncol Biol Phys 2004; 60: 1502–9
- Jiang GL, Liang SX, Zhu XD, Fu XL, Lu HF. Radiation-induced liver disease in three-dimensional conformal radiotherapy for primary liver carcinoma. Abstract. Int J Radiat Oncol Biol Phys 2004; 60(Suppl 1)413
- Fuss M, Thomas CR, Jr. Stereotactic body radiation therapy: An ablative treatment option for primary and secondary liver tumors. Ann Surg Oncol 2004; 11: 130–8
- Herfarth KK, Debus J, Lohr F, Bahner ML, Rhein B, Fritz P, et al. Stereotactic single-dose radiation therapy of liver tumors: Results of a phase I/II trial. J Clin Oncol 2001; 19: 164–70
- Schefter T, Gaspar LE, Kavanagh BD, Ceronsky N, Feiner A, Stuhr K. Hypofractionated extracranial stereotactic radiotherapy for liver tumors. Abstract. Int J Radiat Oncol Biol Phys. 2003; 57(Suppl 1)S282
- Wada H, Takai Y, Nemoto K, Yamada S. Univariate analysis of factors correlated with tumor control probability of three-dimensional conformal hypofractionated high-dose radiotherapy for small pulmonary or hepatic tumors. Int J Radiat Oncol Biol Phys 2004; 58: 1114–20
- Herfarth KK, Debus J. Stereotactic radiation therapy for liver metastases. Chirurg 2005; 76: 564–9
- Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2002; 54: 150–5
- Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 2005; 62: 1371–8