Abstract
CYP2C19*2(G681A), CYP2C19*3(G636A), CYP2D6*4(C188T), CYP2D6*2(C2938T, G4268C), CYP3AP1*3- G44A and CYP3A5*3(A22893G) are the most common polymorphisms detected among Chinese that may influence the efficacy of vinorelbine-based therapies to treat non-small-cell lung cancer (NSCLC). We detected the genotypes of these polymorphisms by PCR-RFLP in 59 patients with NSCLC and assessed their responses to vinorelbine. CYP2D6*4(C188T), CYP3AP1*3 (G -44 A) and CYP3A5*3 were found to be associated with response to vinorelbine. For the 2D6*4 polymorphism, the 18 of 32 (56.25%) patients with homozygous (C/C) responded to this therapy, while 6 of 27 (22.22%) of those heterozygous (C/T) at this site responded. (χ2=5.68, p < 0.05) For the 3AP1*1/*3 polymorphism, 12 of 42 (28.57%) patients with homozygous (A/A) responded, while 12 of 17 (70.59%) with heterozygous (A/G) and homozygous (G/G) responded (χ2=7.19, p < 0.01). CYP3A5*3 polymorphism has a result corresponding to 3AP1*3 polymorphism. Other polymorphisms were not associated with response to vinorelbine. No significant difference in toxicity and survival was observed according to SNP genotype.
Vinorelbine is a semi-synthetic vinca alkaloid that exhibits anti-tumor activity against a wide variety of human tumor cell lines in vitro and in vivoCitation[1]. It is a mitotic inhibitor which has a higher therapeutic index and less neuro toxicity than other vinca alkaloid, and this is related to the fact that it causes less damage to axonal microtubules Citation[2]. Vinorelbine is clinically effective in the treatment of advanced non-small cell lung cancer (NSCLC) and metastasis breast cancer Citation[3–5].
Clinically, vinorelbine shows a large interindividual variability in its response rate to NSCLC. In several large randomized studies of advanced NSCLC patients, those taking vinorelbine-cisplatin attained an overall response rate of 30% to 45%, with median survival time 8 to 10.1 months Citation[6]. This may be caused by its hepatic drug disposition and metabolism.
Cytochrome P450s are heme-containing monooxygenases which play a major role in the biotransformation of anticancer agents in vivo. CYP2C, CYP2D and CYP3A are important and abundantly expressed cytochrome P450 enzymes in liver Citation[7], Citation[8].
Rahmani et al. Citation[9] reported that the catalytic activity CYP3A polymorphism made a major contribution to the overall metabolism of vinorelbine in human liver microsomes. Moreover, CYP2C and CYP2D also participate in the metabolism of vinorelbine Citation[9], Citation[10]. Many genes belonging to these three subfamilies are polymorphic, among which CYP2C19*2(G681A), CYP2C19*3(G636A), CYP2D6*4(C188T), CYP2D6*2(C2938T,G4268C), CYP3AP1*3(G-44A) and CYP3A5*3(A 22893 G) are the most prevalent polymorphisms in Chinese. To investigate whether there is a relationship between specific polymorphisms and clinical response to vinorelbine in NSCLC patients, we chose these seven alleles as subjects. CYP3AP1 is a pseudogene, it was chosen because the CYP3AP1*3 polymorphism has a relationship with CYP3A5*3. Kuehl et al. Citation[11] showed that G/A−44 polymorphism in CYP3AP1 was linked to the splicing defect of CYP3A5*3/*1. Shih et al. Citation[12] also showed that G/A−44 polymorphism of CYP3AP1 promoter has strong association with a/g22893 polymorphism in intron 3 of CYP3A5 in Chinese. Both of them showed that the patients carrying CYP3AP1*1 also carried CYP3A5*1, in the same way, patients carrying CYP3AP1*3 also carried CYP3A5*3 and patients carrying CYP3AP1*1/*3 also carried CYP3A5*1/*3. So we deduced that the genotype of CYP3A5*3 is according to the genotype of CYP3AP1−44 in our study.
Materials and methods
Patients were selected if they met the following criteria: had histologically or cytologically confirmed stage IV or IIIB (with pleural effusion) NSCLC, World Health Organization (WHO) performance status (PS) of 0–1, age > 18 years, and adequate organ function. The ethics committees of participating hospitals approved the protocol, and written informed consent was obtained from each participant.
Patient evaluation
On all patients a complete clinical history and physical examination was performed, including routine hematology and biochemistry analyses, and staging with chest radiographs and computed tomography of the thorax and abdomen. Hematology and biochemistry analyses were repeated at the end of each cycle. Toxicity was classified according to WHO criteria at each cycle for each patient. Response was assessed after two cycles of chemotherapy and every two cycles thereafter, using Response Evaluation Criteria in Solid Tumor Group (RECIST) guidelines.
Patients received intravenous doses of cisplatin 75 mg/m2 plus vinorelbine 25 mg/m2 on day 1, day 8, every 3 weeks.
Sample collection and SNP genotyping
Venous blood (1 ml) was collected from each subject and placed into tubes containing 3.2% citric sodium.
Genomic DNA was isolated with a DNA Blood isolation kit (Omega, America), according to the manufacturer's instructions.
CYP genotypes were performed by PCR-RFLP. Fragments containing the polymorphisms 2C19*2, 2C19*3, 2D6*4, 2D6*2, CYP3AP1*3 were amplified with PCR primers designed based on CYP sequence from Genbank. The primers were listed in . PCR reactions were carried out in 50 µl of solution consisting of 5 µl of 10×PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.4 µM of each primer, less than 1 µg of genomic DNA as template, and 2.5 U of Taq polymerase (TaKaRa). The PCR reactions were performed in the program of 94°C 3 min, 94°C 30 s, 50–65°C 30 s, 72°C 1 min, repeated 30 cycles and at last prolonged at 72°C for 5 min. The annealing temperature and the restriction enzymes corresponding to the polymorphism sites were listed in .
Table IA. Primer sequence ,anneal temperature and length of PCR fragment.
Table IB. The restriction enzymes correspond to the polymorphism sites.
Statistical Analysis
χ2 test was used to summarize the association of response to vinorelbine with CYP polymorphisms. The proportion of response (CR and PR), odds ratio (OR) and 95% CI were calculated. The Hardy-Weinberg equilibrium of alleles at individual loci was tested with a goodness-of-fit χ2 test with one degree of freedom to compare the observed genotype frequencies with the expected genotype frequencies among the subjects.
Results
Baseline of the patients
A total of 59 patients were enrolled in this study. The baseline characteristics and response for the 59 patients are shown in . Median age was 55 years; 71% were male. The overall response including complete response (CR) and partial response (PR) to vinorelbine was 40.67% (24 of 59; S = 0.0639, 95% CI 28.13%, 53.21%) while 59.33% (35 of 59; 95% CI 46.79%, 71.87%) had stable disease or progressive disease and no change.
Table IIA. Baseline characteristic and response for all 59 patients.
Single nucleotide polymorphism (SNP) genotype distribution, allele frequencies and test of Hardy-Weinberg equilibrium
The distributions and frequencies detected of adopted SNPs were calculated (as seen in ). The genotype distributions of both polymorphisms among the overall cases were in Hardy-Weinberg equilibrium.
Table IIB. Single nucleotide polymorphism(SNP) genotype distribution and allele frequencies.
Comparison of response rate according to baseline characteristics of patients
To test whether various clinical characteristics contribute to response rate, patients were grouped according to age, gender, histology and phase. Response rates were compared among different groups. There were no significant differences in response rates according to any factor (as seen in ).
Table III. Comparison of response rate according to baseline characteristics of patients.
There was no significant association between the six polymorphisms and age, gender, histology or phase.
Response and SNP genotyping
A partial response was attained in 24 patients (40.68%). Response rate according to CYP2D6*4 was 56.25% for 32 patients homozygous for the wild-type C/C, and it was 22.22% for 27 patients heterozygous for C/T and homozygous for the rare allele T/T (p = 0.016). Response rate according to CYP3AP1 was 28.57% for 42 patients homozygous for the wild-type A/A, while it was 70.59% for 27 patients heterozygous for A/G and homozygous for the rare allele G/G (p = 0.004). In the same way, response rate according to CYP3A5*3 was 70.59% for 27 patients heterozygous for A/G and homozygous for the wild allele A/A, while it was 28.57% for 42 patients homozygous for the rare allele G/G (p = 0.004). There were no significant differences in response according to SNP genotype of CYP2C19*2, CYP2C19*3 and CYP2D6*2 ()
Table IV. Response according to SNP genotypes.
Survival and SNP genotyping
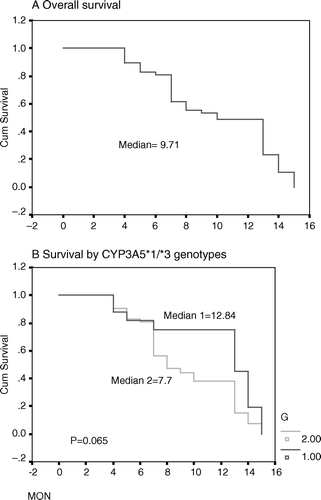
shows median survival overall and according to SNP genotype. Overall median survival was 9.71 months. Median survival according to CYP3A5 genotype was 12.84 months for 17 patients with homozygous or heterozygous wild-type genotypes considered as one group and 7.7 months for 42 patients homozygous for the rare alleles G/G (p = 0.065). No significant differences in median survival were observed according to CYP3A5*3 SNP genotype and other SNPs genotypes.
Toxicity and SNP genotyping
All patients were included in the toxicity analysis and the overall data were listed in . Grade ≥I and grade ≥II toxicity according to SNP genotypes were listed in . Toxicity is evaluated according to the ECOG toxicity criteria including anemia, leucopenia, thrombocytopenia and nausea/vomiting. No grade IV toxicity was observed in this investigation. No significant differences were found among SNP genotypes.
Table VA. The overall analysis of toxicity of 59 patients.
Table VB. Toxicity according to single nucleotide polymorphism genotype (presented as percent).
Discussion
In our study we assessed seven common polymorphisms of the CYP450 gene that may influence the response rate of vinorelbine.
We found that a significant relationship exists between clinical response to vinorelbine chemotherapy and the 2D6*4 polymorphism of the CYP450 gene. Patients with the C/C genotype were 2.53 times more likely to have CR or PR compared with the C/T genotype group. No toxicity difference was detected according to the SNP genotype.
Wang et al. Citation[13] reported that C188→T mutation caused pro34→ser mutation, and the shift of the amino acid caused the decrease of CYP2D6. In theory, patients with C/T heterozygous and T/T rare allele homozygous have lower CYP2D6 activity and should dispose and metabolize less of its substrates, the substrates should have higher tissue concentrations and higher response rate to target tumor cells. However the results we observed were contrary to what we expected.
According to the genotype of CYP3AP1−44, we can deduce the genotype of CYP3A5*3.Patients with wild allele A (including A/A homozygous and A/G heterozygous) were 2.47 times more likely to have CR or PR compared with patients with the homozygous rare allele G/G on this site.
Kuehl et al. Citation[11] reported that individuals with at least a wild allele in the CYP3A5*3 expressed CYP3A5 normally; those with homozygous rare alleles didn't express CYP3A5 at all. In theory, the homozygous rare alleles should metabolize and dispose less substrates of CYP3A5 and have higher concentration in target tumor cells, but the results we observed were also not this case.
It is a novel finding which deviates far from what we imagined when we designed this study. We only focused on drug metabolism, but the full spectrum of drug disposition, including a growing list of transporters that influence drug absorption, distribution, and excretion should also be taken into account. Expression of CYP450 is also regulated by other genes such as PXR and CAR Citation[11], Citation[14], Citation[15]. The outcome may also be influenced by the polymorphisms of these genes. But the mechanisms of interindividual differences are far from being clarified.
Moreover, we analyzed the toxicity and survival data of all 59 patients. According to our investigation, the response rate of vinorelbine-cisplatin chemotherapy is about 40%, the median survival time is 9.71 months and the 1-year survival rate is about 40%. All these data are consistent with those of other groups such as TAX326 Citation[16], Italian Lung Cancer Project Citation[17]. We also associated with CYP genotypes, but no difference in toxicity and survival was observed according to the CYP SNP genotypes.
There are a few issues that must be considered when evaluating the findings of the current study. The number of patients in this study is relatively small. Thus, we can't say for sure that these three polymorphisms can be regarded as markers of chemotherapy outcome of vinorelbine on NSCLC but others can't. After all, as to 2C19*2(G681A), 2D6*2 (C2938T) and 2C19*3 (G636A) polymorphisms, although no significant association was observed, the tendency is distinct. If the number of patients is large enough, the result may be different. For this reason, a larger prospective study will be needed to confirm the findings of this study.
In this study Dr. Shengwei in Shandong Province Hospital provided part of the samples and clinic materials. The research was supported by National Natural Science Fund of China, No.30471979.
References
- Ashizawa T, Miyoshi K, Asada M, Kobayashi E, Okabe M, Morimoto M, et al. Anti-tumor activity of Navelbine (vinorelbine ditartrate), a new vinca alkaloid analog. Jpn J Cancer Chemother 1993; 20: 59–66
- Binet S, Fellous A, Lataste H, Krikorian A, Couzinier JP, Meininger V. In situ analysis of the action of Navelbine on various types of microtubules using immuno-fluorescence. Semin Oncol 1989; 16(Suppl 4)5–8
- Goa KL, Faulds D. Vinorelbine: A review of its pharmacological properties and clinical use in cancer chemotherapy. Drugs Aging 1994; 5: 200–34
- Zhou XJ, Rahmani R. Preclinical and clinical pharmacology of vinca alkaloids. Drugs 1992; 44(Suppl 4)1–16
- Toso C, Lindley C. Vinorelbine: A novel vinca alkaloid. Am J Health-Syst Pharm 1995; 52: 1287–304
- Pallis, AG, Agelidou, A, Papakotoulas, P, Tsaroucha, A, Agelidou, M, Agelaki, S, et al. A multicenter phase II study of sequential vinorelbine and cisplatin followed by docetaxel and gemcitabine in patients with advanced non-small cell lung cancer. Lung Cancer 2006;52:165–71. Epub 2006 Mar 24.
- Krikorian A, Rahmani R, Bromet M, Bore P, Cano JP. Pharmacokinetics and metabolism of Navelbine. Semin Oncol 1989; 16(Suppl 4)21–5
- Hashimoto H, Toide K, Kitamura R, Fujita M, Tagawa S, Itoh S, et al. Gene structure of CYP3A4, an adult-specific orm of cytochrome P450 in human livers and its transcriptional control. Eur J Biochem 1993; 218: 585–95
- Zhou XJ, Zhou-Pan XR, Gauthier T, Placidi M, Maurel P, Rahmani R. Human liver microsomal cytochrome P450 3A enzymes mediated vindesine biotransformation: Metabolic drug interactions. Biochem Pharmacol 1993; 45: 853–61
- Zhou-Pan XR, Seree E, Zhou XJ, Placidi M, Maurel P, Barra Y, et al. Involvement of human liver cytochrome P450 3A in vinblastine metabolism: Drug interactions. Cancer Res 1993; 53: 5121–6
- Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet 2001; 27: 383–91
- Shih PS, Huang JD. Pharmacokinetics of midazolam and 1′-hydoxoymidazolam in Chinese with different CY3A5 genotypes. Drug Metab Dispos 2002; 30: 1491–6
- Wang SL, Huang JD, Lai MD, Liu BH, Lai ML. Molecular basis of genetic variation in debrisoquine hydroxylation in Chinese subjects: Polymorphism in RFLP and DNA sequence of CYP2D6. Clin Pharmacol Ther 1993; 53: 410–8
- Masuyama H, Suwaki N, Tateishi Y, Nakatsukasa H, Segawa T, Hiramatsu Y. The pregnane X receptor regulates gene expression in a ligand- and promoter-selective fashion. Mol Endocrinol 2005; 19: 1170–80
- Zhang J, Huang W, Qatanani M, Evans RM, Moore DD. The constitutive androstane receptor and pregnane X receptor function coordinately to prevent bile acid-induced hepatotoxicity. J Biol Chem 2004; 279: 49517–22
- Fossella F, Pereira JR, von Pawel J, Pluzanska A, Gorbounova V, Kaukel E, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: The TAX 326 study group. J Clin Oncol 2003; 21: 3016–24
- Scagliotti GV, De Marinis F, Rinaldi M, Crino L, Gridelli C, Ricci S, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 2002; 20: 4285–91