Abstract
The purpose was to evaluate the clinical results of stereotactic radiotherapy in primary liver tumors and hepatic metastases. Five patients with primary liver cancer and 39 patients with 51 hepatic metastases were treated by stereotactic radiotherapy since 1997. Twenty-eight targets were treated in a “low-dose”-group with 3×10 Gy (n = 27) or 4×7 Gy (n = 1) prescribed to the PTV-encl. 65%-isodose. In a “high-dose”-group patients were treated with 3×12 − 12.5 Gy (n = 19; same dose prescription) or 1×26 Gy/PTV-enclosing 80%-isodose (n = 9). Median follow-up was 15 months (2–48 months) for primary liver cancer and 15 months (2–85 months) for hepatic metastases. While all primary liver cancers were controlled, nine local failures (3–19 months) of 51 metastases were observed resulting in an actuarial local control rate of 92% after 12 months and 66% after 24 months and later. A borderline significant correlation between dose and local control was observed (p = 0.077): the actuarial local control rate after 12 and 24 months was 86% and 58% in the low-dose-group versus 100% and 82% in the high-dose-group. In multivariate analysis high versus low-dose was the only significant factor predicting local control (p = 0.0089). Overall survival after 1 and 2 years was 72% and 32% for all patients and was impaired due to systemic progression of disease. No severe acute or late toxicity exceeding RTOG/EORTC-score 2 were observed. Stereotactic irradiation of primary liver cancer and hepatic metastases offers a locally effective treatment without significant complications in patients, who are not amenable for surgery. Patient selection is important, because those with low risk for systemic progression are more likely to benefit from this approach.
The liver is second only to regional lymph nodes as a site for metastatic disease. At autopsy 25–50% of patients dying from cancer have liver metastases Citation[1]. For patients with diagnosed liver metastases live expectancy without treatment is poor with about 5 months Citation[2]. Surgical data showed that a subgroup of patients benefits from aggressive local treatment. Under certain circumstances - e.g. solitary liver metastasis of colorectal cancer - surgical resection of liver metastases can lead to 5-year survival rates of up to 30% Citation[2–4]. Non-surgical approaches such as thermal ablation techniques have been developed during the last years and these techniques have also confirmed the efficacy of local treatment Citation[5–7].
During the last years, the significance of whole liver irradiation as palliative treatment has declined due to more effective chemotherapy regimes. Only a few centers have kept radiotherapy within treatment protocols Citation[8], Citation[9]. Nevertheless recent developments in radiotherapy as three dimensional (3-D) treatment planning, breathing-control techniques and image guidance have introduced a potential for high precision and dose-escalated focal irradiation. The advantage of radiotherapy is the non-invasive approach and the biological pathway of cell damage; while surgical and thermoablative techniques have to respect the wall of intrahepatic vessels or biliary ducts to avoid immediate necrosis or leakage, radiotherapy will lead to a delayed and partly selective tumor cell necrosis.
Blomgren and Lax from Karolinska Hospital in Stockholm, Sweden, published encouraging results of stereotactic radiotherapy for liver tumors in 1995 and updated their results in 1998 Citation[10]. In the follow-up to now only limited data have been published on stereotactic irradiation of liver targets, although the results seemed promising Citation[11–14].
In Wuerzburg stereotactic body radiotherapy (SBRT) has been performed since 1997 not only for liver tumors but also for pulmonary, abdominal and pelvic targets Citation[15], Citation[16]. In this communication the treatment results in 39 patients with 51 liver metastases and five patients with primary liver cancer are reported with a maximum follow-up time of 85 months.
Materials and methods
The introduction of SBRT in our department was based on the method described by Blomgren and Lax Citation[10], Citation[17], Citation[18]. The patient is immobilized in a vacuum pillow, which is firmly attached to a stereotactic body frame (SBF; ELEKTA-Instr., Stockholm). This allows for patient fixation and fiducial markers in the frames' sidewalls are used as a system of coordinates. Because the liver continuously moves with breathing abdominal compression for reduction of breathing mobility was applied in all patients. For that purpose a template is pressed into the patient abdomen by a scaled screw, which is attached to the SBF by a flexible arc. Using this technique the target mobility was reduced to 5–8 mm in superior-inferior direction. Details of this technique and its precision have been published previously Citation[19], Citation[20].
Patient selection followed the ethical rules of Helsinki and the patients’ informed consent was mandatory. Estimated patients’ life expectancy should be more than 6 months with liver function test close to normal and no presence of ascites, jaundice or impaired blood coagulation. Standard treatment as surgical resection had to be inappropriate according to a surgeon′s statement. Extrahepatic tumor had to be controlled or treated. Hepatic staging was performed by MRI- or multiphase contrast-enhanced CT-scan, since 2003 a PET-scanner was also available. Target size was no primary exclusion criterion defined, but no more than 50% of the total functional liver tissue should receive more than 5 Gy and no more than 30% more than 7 Gy in each of 3 fractions of 10–12.5 Gy. The estimated dose to serial organs at risk as the wall of the stomach, duodenum, small or large bowel must not exceed a fraction dose of more than 7 Gy in a 5 cm3 volume. Additional chemotherapy prior to or following SBRT was allowed with a minimum interruption of at least 6 weeks after treatment.
Breathing mobility of the target (not only surrogate markers such as the hepatic dome) was evaluated: dynamic CT scans were acquired at the target level without table movement over a period of 6 s, later of 15 s with one image per second. The abdominal pressure was adjusted to achieve a maximum of breathing mobility of 5–8 mm. Then a multiphase iv-contrast enhanced CT-scan was performed in the arterial, portal-venous and venous phase with a maximum slice thickness of 5 mm. The scan covered the whole liver plus 2–5 cm cranio-caudal (depending on the target position) to allow eventual use of non-coplanar beams and to include relevant organs at risk. After completion of the study the patient was released from the frame. Treatment was scheduled usually a few days later.
Target definition was performed by contouring the CTV in the contrast-phase with the largest tumor diameter. The CTV was assumed to be the contrast enhancing zone plus 3 mm. The PTV was defined by adding a security margin of 5 mm in transversal and 5–10 mm in longitudinal direction with respect to breathing mobility using an automated tool of the 3D-treatment planning System Helax-TMS® versions 4.01A, 4.01B, 5.1 and 6.1A (Theranostic B.V., Veenendaal, The Netherlands). The reference isodose was to cover at least 95% of the PTV with 6 or 18 MV photons. Dose distribution was calculated based on a pencil beam algorithm, which proved to be reliable for intrahepatic tumors Citation[21]. Conformality was achieved by a minimum of five static or rotational beams, occasionally supported by non-coplanar fields. Details of dose planning and conformity of dose distribution have been reported previously Citation[20], Citation[22].
Initially dose prescription followed the practice as published by Blomgren and Lax: the liver tumors were irradiated with 3×10 Gy, normalized to the PTV-enclosing 65%-isodose resulting in a 50% higher maximum dose (100% = 15 Gy) close to the isocenter. Preliminary treatment results of the first 24 targets have been reported previously in 2001 Citation[22], they are included in this evaluation in an updated form. Since November 2001 the dose was increased to 3×12.5 Gy following the same prescription rules after eight local failures of 28 targets had been observed. Additionally single dose treatment with 1×26 Gy, normalized to the PTV-enclosing 80%-isodose (=32.5 Gy/isocenter) was introduced after publication of the Heidelberg results by Herfarth et al. Citation[12]. Only small tumors with a PTV not exceeding 50 cm3 and without central location in the liver were considered suitable for single dose radiosurgery. Details of patient, target and treatment characteristics are shown in .
Table I. Patient-, target- and treatment-characteristics of 5 patients with primary liver cancer and 39 patients with 51 hepatic metastases (related to targets)
To allow for comparison of dose a biological equivalent dose (BED) was calculated according to the formula BED(Gy)=dose/fraction×fraction number (1 + fraction dose/a/ß) using an alpha/beta of 10 Gy for tumor tissue despite general uncertainty, whether the use of the LQ-model will be reliable for such high fraction doses. The BED at the isocenter/PTV-margin are as follows: 138/94 Gy (1×26 Gy), 169/84 Gy (3×12.5 Gy), 151/79 Gy (3×12 Gy), 117/60 Gy (3×10 Gy) and 90/48 Gy (4×7 Gy).
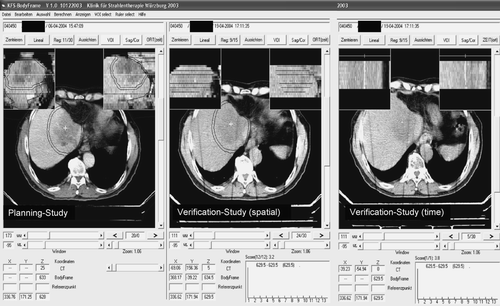
Prior to irradiation the patient was re-positioned in the SBF. CT-verification to ensure reproducible reduction of breathing mobility and correctness of the stereotactic coordinate relative to the target was performed prior to each treatment fraction. Until January 2002 this procedure had to be performed at a CT-scanner outside of the treatment room, which required patient transportation in the SBF. Since February 2002 isocenter verification was performed directly at the linac using a carbon fiber treatment table and a mobile CT-scanner. The CT was aligned after a isocentric 90 degree couch rotation. The correct isocenter position in the target was evaluated and corrected by the use of dedicated in-house software Citation[24] (). Since September 2005 this procedure is performed by an integrated system of a linac, a cone-beam CT and an evaluation Software provided by the ELEKTA-Synergy S accelerator.
Figure 1. CT-verification on the treatment couch using in-house software. The planning study and the verification study are matched using the fixed stereotactic system represented by fiducials in the body frames sidewalls. The isocenter coordinate is automatically placed at the planned position and can be moved by mouse click from the planned to the appropriate position in the verification study. The new coordinate is shown in the bottom window. The structure set of the planning study (CTV, PTV, liver contour etc.) are related to the isocenter coordinate and can be moved with the isocenter to the most appropriate position. Because the verification study (spatial) is just a slice-by-slice scan the isocenter correction might be performed in a random position of the breathing cycle. Therefore a second (time) study is performed scanning 15 s continuously at the chosen longitudinal couch position. During that time the influence of breathing mobility can be evaluated. In the shown case no correction is necessary: in the two small reconstructions at top of the right window a sagittal and coronal view are shown. The 15 slices are representing the 15 s breathing scan at a position marked by a radioopaque clip from a former liver resection. The clip is not moving to the left or right nor to the anterior-posterior direction indicating no relevant breathing mobility of the target.
Treatment was usually performed on an out patient basis. The fractions were applied in a 2–3 days interval (e.g. Monday – Wednesday - Friday). The first patients received liver function tests and prophylactic medication for fever, chills or pain, which had been reported to occur in about 25–30% of cases. This practice was abandoned due to unchanged lab findings during the treatment phase and the low intensity and spontaneous remission of acute side effects.
Evaluation of treatment results
Five patients with medically inoperable primary liver cancer (HCC n = 4; CCC n = 1) and 39 patients with 51 hepatic metastases (CRC n = 24; breast n = 11, misc. n = 17) have been treated until October 2005.
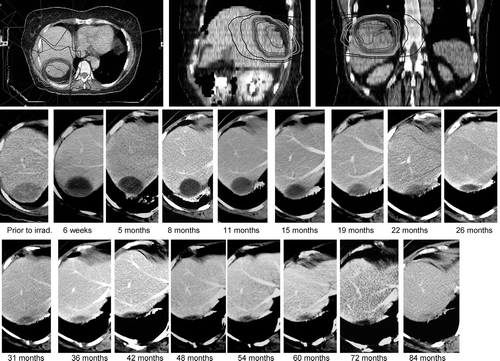
Treatment results and side effects were prospectively evaluated by clinical examination and CT- or MRI-scans 6 weeks after irradiation and then followed by further examinations every 3 months. An example of a dose distribution and follow-up is shown in (). Primary endpoint was local tumor control defined as tumor shrinkage or no tumor progress (volume reduction of >25% of pre-therapeutic size) during follow-up. Local failure was defined as tumor progression after therapy (increase of volume compared to pre-therapeutic size) or re-growth after initial shrinking. To differentiate between tumor re-growth and radiogenic inflammation with a contrast enhancing zone, tumor recurrence was considered continuous mass increase during follow-up. The morphological changes of normal liver and target tissue after stereotactic irradiation have been described in detail by Herfarth et al. Citation[25]. If such a re-growth was diagnosed on consecutive CT-scans, the date of recurrence was determined as the first date of a CT-abnormality. Secondary endpoints were treatment related acute and late toxicity evaluated according to the RTOG/EORTC-score, freedom from systemic progression and overall survival.
Figure 2. Dose distribution and follow-up of a 62 year old female treated for a solitary liver metastasis of ovarian cancer progressing under second line chemotherapy. The CTV was 154 cm3, the PTV was 300 cm3. The treatment was performed by 3×10 Gy to the PTV-enclosing 65%-isodose. The target gets necrotic 6 weeks after irradiation (a mirror line can be seen), the liquid cyst of necrotic tumor is resorbed over the years. After 5 months an asymptomatic fibrosis of adjacent lung tissue can be observed.
For statistical evaluation of time-event analyses the Kaplan-Meier method and the log-rank-test were used. For analysis of factors with potential influence on local tumor control (independent variable) a multivariate analysis using a multiple regression model was performed. Factors included were the size of the target volume (CTV and PTV), the magnitude of the security margin (PTV/CTV-ratio), a chemotherapy prior or after radiotherapy, the dose (“low-dose” as 3×10 Gy/4×7 Gy versus “high-dose” as 3×12 − 12.5 Gy, 1×26 Gy) and histology of the primary tumor (colorectal cancer versus non-CRC). For statistical analyses Statistica Software (Statistica version 6.1; StatSoft) was used Citation[26].
Results
Primary liver cancer
Median follow-up was 15 months (2–48 months, mean 16 months). No local failure was observed. While one patient with a HCC is alive after 48 months and another after 15 months, the other three patients died of multifocal tumor progression in the liver after 2, 7 and 17 months. No relevant acute and or late side effects of irradiation were observed.
Liver metastases
Median follow-up was 15 months (2–85 months) for all patients and 21 months (2–85 months) for surviving patients. Nine local failures were observed leading to a crude local control rate for all targets of 82%. The local failures occurred 3, 8, 9, 14, 3×16, 17 and 19 months after treatment, median time to local failure was 16 months. The corresponding actuarial local control rate was 92% after 12 months and 66% after 24 months. While two local failures (ovarian cancer, breast cancer) have to be considered as marginal the other seven local failures were in-field (kidney cancer n = 1; colorectal cancer n = 6 ().
Figure 3. Actuarial local control of liver metastases, depending on the primary tumor. After two years the local control rate of metastases of colorectal cancer appeared to be lower compared to metastases of other primaries. The difference is statistically not significant, but consistent with findings of other authors Citation[30].
![Figure 3. Actuarial local control of liver metastases, depending on the primary tumor. After two years the local control rate of metastases of colorectal cancer appeared to be lower compared to metastases of other primaries. The difference is statistically not significant, but consistent with findings of other authors Citation[30].](/cms/asset/0e82b7ad-213b-4035-bca9-c8a2d8694479/ionc_a_190402_f0003_b.gif)
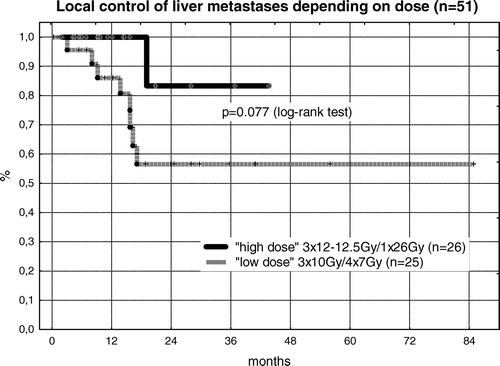
Eight local recurrences were seen in the low-dose group (n = 25) with a prescription dose of 3×10 Gy. Of 12 CRC-patients treated in the in this low dose group, half of them (n = 6) suffered local failure. In the high dose group (n = 26) with prescription doses of 3×12 − 12.5 Gy or 1×26 Gy only one marginal recurrence (breast cancer) was observed. Of 11 CRC-patients included in the high-dose group, no local failure was recorded. While actuarial local control of the high dose group was 100% after 12 months and 82% after 24 months, the corresponding local control rates for the low dose group were 86% and 58%, respectively (p = 0.077; ).
Figure 4. The first series of patients from 11/1997 to 10/2001 was irradiated by 3×10 Gy (n = 24) and in one case by 4×7 Gy, all prescribed to the PTV-enclosing 65%-isodose. After 8 local failures were observed in this group, the dose was increased to 3×12 Gy (n = 5) and 3×12.5 Gy (n = 14) using the same dose prescription. Additionally single dose treatment was introduced with 1×26 Gy prescribed to the PTV-enclosing 80%-isodose. Although the difference is not significant in log-rank test so far, only one local failure was observed in the higher dose group indicating the role of dose escalation even in the high-dose stereotactic treatment approach.
The importance of dose for achieving local control is supported by the results of multivariate analysis. Multiple regression revealed dose (“low”- versus “high”-dose) as the only significant factor for achieving tumor control (p = 0.0089). The other factors as volume of CTV, PTV, PTV/CTV-ratio, chemotherapy and histology (CRC versus non-CRC) appeared to be not relevant in this setting.
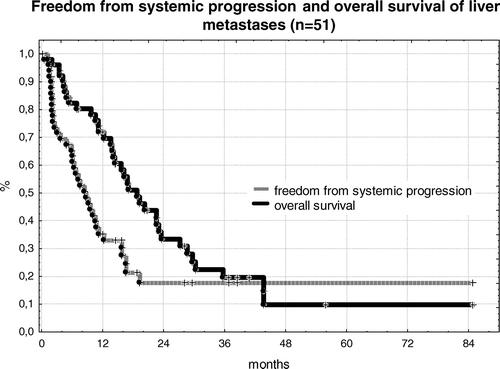
Overall survival of patients was 72% after 12 months, 32% after 24 months and 22% after 36 months. Survival was compromised due to systemic progression of disease. Freedom from systemic progression was only 35% after 12 months and 19% after 24 months and later. The Kaplan-Meier plots of overall survival and systemic progression of disease are shown in . Median time to systemic progression of disease was 8 months; median overall survival of patients was 16 months.
Toxicity
Overall toxicity was mild. Regarding early toxicity 73% of patients (n = 41) were without any clinical side effect. In 14% (n = 8) mild symptoms as minor pain, fever and chills not requiring any intervention (grade 1) were observed. These symptoms typically started shortly after treatment and lasted for a few hours. In seven patients (13%) these symptoms required treatment (grade 2) with analgetics (metamizol) or stereoids (methyl-prednisolon 8 mg po tid) for a week). Three patients with targets located in close relation to the stomach and duodenal wall were treated prophylactically with ranitidin 150 mg bid or omeprazol 20 mg qd for six weeks for protection of peptic ulcer.
No late toxicity clearly related to the irradiation has been observed. In one patient with two sequentially irradiated targets close to the liver hilum signs of liver fibrosis, portal hypertension, ascites and bleeding from esophageal varices were described 41 months and 28 months after irradiation. However, because of new metastasis in that area the causal relation is not clear. One patient treated for a metastasis (3×10 Gy, PTV 67 cm3) very close to the thoracic wall complained of pain 4 months after irradiation, which required analgetic medication and potentially was due to irritation of an intercostal nerve. Five targets were located very closely to the vena cava inferior and about half of the vessels‘ circumference received the prescription dose. No damage of the vessel, induction of thrombi to its wall or signs of occlusion were observed.
All patients showed changes in contrast enhanced CT-scans during follow-up. Usually a hypodense area was visible encompassing the target and corresponding approximately to the cumulative 20 Gy-isodose in fractionated treatment. This reaction is potentially due to a form of veno-occlusive disease in that area, but was not associated with changes in overall liver function. During follow-up this zone of hypodensity shrunk continuously in all cases studied. At the superficial margin of the target a contrast enhancing zone was visible in most cases 3 to 6 months after treatment, enclosing a hypodense area, which is assumed to be tumor necrosis.
Discussion
Within this study local control of a group of patients with intra-hepatic tumors treated by stereotactic radiotherapy is reported. Due to the small number of five patients treated for primary liver cancer no firm conclusions can be drawn for this group. Discussion concentrates on treatment results for the 39 patients with 51 hepatic metastases. Regarding published data this is the second largest collective so far. Herfarth et al. reported the results of four patients with primary liver cancer and 37 patients with 56 hepatic metastases Citation[12]. An overview of published results is given in . Experience in stereotactic treatment of liver tumors is limited, especially if compared to surgery and thermoablative approaches Citation[2–7]. Despite this fact stereotactic radiotherapy might offer a valuable option for selected patients, because it is the only non-invasive treatment approach and therefore can be offered to patients not amenable for invasive or minimal invasive interventions.
Table II. Published results of stereotactic irradiation of hepatic targets. (The results of the previous publication of Wulf et al. are included in the presented data in an updated form. The results published by Blomgren et al. in 1995 Citation[9] are included in the publication of 1998). (Abbr.: HCC/CCC: hepatocellular/cholangiocellular carcinoma; Metas: metastases; CRC: colorectal cancer).
Due to the small number of targets treated with stereotactic radiotherapy most authors report local control as crude data. In the presented group of patients the crude local control rate was 82% compared to 78%–100% reported in the literature. A borderline significant correlation between treatment dose and local control was seen (p = 0.077): crude local control of the first 25 targets treated by 3×10 Gy (n = 24) or 4×7 Gy (n = 1) was only 68% (17/25 targets). For treatment with escalated doses of 3×12 − 12.5 Gy (n = 18) or 1×26 Gy (n = 8) an excellent crude local control rate of 96% (25/26 targets) was seen. Median follow-up was not different between these two groups and therefore should not be responsible for the observed difference. Prescribed dose was found to be the only significant factor for local control in multivariate analysis. The fact that the majority of the local recurrences in the low dose group were “in-field” recurrences (6/8) further supports the hypothesis of a dose response relation. For “in-field” recurrences a geographical miss or suboptimal target definition is less likely than for failures at the margin of the treatment volume. Insufficient treatment dose or decreased radiation sensitivity is therefore the most likely explanation for these “in-field” local failures in the low-dose group. A correlation between dose and local control has already been described for stereotactic radiotherapy of lung tumors (primary NSCLC and metastases) Citation[15], Citation[27–29].
For stereotactic treatment of liver metastases a dose dependency has also been observed by Herfarth et al., who started radiosurgery with 1×14 Gy/isocenter and then increased dose to 1×26 Gy/isocenter. Among six targets treated with doses less than 20 Gy three local recurrences occurred. Although the authors interpreted these failures as “learning curve”, insufficient dose might be another explanation Citation[12]. In an update of their data with a total of 70 targets and a median follow-up of 15 months Herfarth et al. not only reported late recurrences even 4 years after irradiation, but also found a significantly decreased local control rate in metastases of colorectal cancer: the local control rate after 18 months was only 45% in 35 metastases of CRC compared to 91% in patients with other primaries (p < 0.001) Citation[30]. Furthermore, patients with metastases of CRC, who underwent previous chemotherapy with oxaliplatin or CPT-11, showed a significantly impaired local control rate (p < 0.01). While an influence of chemotherapy on local control was not seen in our patients, the apparently reduced control rate of metastases from colorectal cancer is shown also in this series (however not at level of statistical significance).
As a consequence of these findings further dose escalation in this patient group might be discussed. An increased PTV-dose has to be balanced against the risk of side effects. Previous publications as well as this communication have not reported serious toxicity. Only Blomgren as the pioneer of stereotactic radiotherapy in extracranial targets described serious side effects in five patients (4 fatal) from the early phase of clinical implementation. Three of these patients had preexisting, clinical manifest liver cirrhosis. In another patient with two simultaneously treated subcapsular HCC (CTV 622 cm3 and 15 cm3) rapid tumor cell necrosis was associated with fatal peritonitis 2 weeks after irradiation. One patient developed hemorrhagic gastritis 2 weeks after irradiation of about 30% of the ventricular wall with 2 fraction doses of 7 Gy Citation[10]. No serious toxicity was observed neither in patients treated later nor in the reports of other authors. The risk for severe side effects due to high dose treatment seems to be low, if irradiated volume, preexisting liver disease or dose to the wall of adjacent hollow organs are taken into account.
Recently, Schefter et al. published the results of a multicenter phase I dose escalation trial for stereotactic radiotherapy of liver tumors in patients without pre-existing liver disease Citation[13]. The study was started at a level of 3×12 Gy prescribed to the PTV-enclosing 80–90%-isodose. The dose was increased in 2 Gy-increments per fraction after three patients were treated without dose limiting toxicity (DLT) or after six patients, if one experienced a DLT. The normal tissue dose constraints stated that at least 700 cm3 of normal liver tissue should receive a cumulative dose of <15 Gy and the point dose to the stomach or intestinal wall should not exceed 10 Gy/fraction. The trial was closed after treatment of 18 patients with a total of 24 targets at a level of 3×20 without reaching DLT. Despite the fact that the targets were comparable small (median CTV 18 cm3, 3–98 cm3) () the trial demonstrates the potential of further dose escalation without unacceptable toxicity. The results for local tumor control have not been published so far, probably due the short median follow-up of 7 months (4–19 months).
In summary the presented data and published results demonstrate a potential for lasting local control achieved by stereotactic treatment of intrahepatic malignancy. The method seems to be safe concerning both acute and late toxicity, if restrictions to normal tissue dose and patient selection are respected. Nevertheless the most appropriate dose and fractionation scheme has not been determined, yet. The presented data suggest a dose-response relation with decreased local tumor control rates, if lower doses than 3×12 Gy, normalized to the PTV-enclosing 65%-isodose (18 Gy/isocenter) are prescribed.
References
- Edmondson HA, Peters RL. Neoplasms of the liver. Diseases of the liver, L Schiff, E Schiff. J.B. Lippincott, Philadelphia 1987; 1109–58
- Lehnert T, Golling M. Indikationen und Ergebnisse der Lebermetastasenresektion. Radiologe 2001; 41: 40–8
- Fong Y, Cohen AM, Forter JG, et al. Liver resection for colorectal metastases. J Clin Oncol 1997; 15: 938–46
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer. Ann Surg 1999; 230: 309–21
- Vogl T, Straub R, Eichler K, et al. Colorectal carcinoma metastases in liver: Laser-induced interstitial Thermotherapy - Local tumor control rate and survival data. Radiology 2004; 230: 450–8
- Solbiati L, Livraghi T, Goldberg SM, et al. Percutanous radio-frequency ablation of hepatic metastases from colorectal cancer: Long-term results in 117 patients. Radiology 2001; 221: 159–66
- Livraghi T, Bolondi L, Lazzaroni S, et al. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. A study on 207 patients. Cancer 1992; 69: 925–9
- Dawson LA, McGinn CJ, Normolle D, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol 2000; 18: 2210–8
- Park HC, Seong J, Han KH, et al. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2002; 54: 150–5
- Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Acta Oncol 1995; 34: 861–70
- Blomgren H, Lax I, Göranson H, et al. Radiosurgery for tumors in the body: Clinical experience using a new method. J Radiosurgery 1998; 1: 63–74
- Herfarth KK, Debus J, Lohr F, et al. Stereotactic single dose radiation therapy of liver tumors: Results of a phase I/II trial. J Clin Oncol 2001; 19: 164–70
- Schefter T, Kavanagh B, Timmerman R, et al. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 2005; 62: 1371–8
- Gunvén P, Blomgren H, Lax I. Radiosurgery for recurring liver metastases after hepatectomy. Hepato-Gastroenterology 2003; 50: 1201–4
- Wulf J, Haedinger U, Oppitz U, et al. Stereotactic radiotherapy of primary lung cancer and pulmonary metastases: A non-invasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys 2004; 60: 186–96
- Wulf J, Haedinger U, Oppitz U, et al. Stereotactic boost irradiation for targets in the abdomen and pelvis. Radiother Oncol 2004; 70: 31–6
- Lax I. Target dose versus extra-target dose in stereotactic radiosurgery. Acta Oncol 1993; 32: 453–7
- Lax I, Blomgren H, Näslund I, et al. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol 1994; 33: 677–83
- Wulf J, Hädinger U, Oppitz U, et al. Stereotactic radiotherapy of extracranial targets: CT-simulation and accuracy of treatment in the stereotactic body frame. Radiother Oncol 2000; 57: 225–36
- Wulf J, Haedinger U, Oppitz U, et al. Impact of target reproducibility on tumor dose in stereotactic radiotherapy of targets in the lung and liver. Radiother Oncol 2003; 66: 141–50
- Haedinger U, Krieger T, Flentje M, et al. Influence of the calculation model on dose distribution in stereotactic radiotherapy of pulmonary targets. Int J Radiat Oncol Biol Phys 2005; 61: 239–49
- Haedinger U, Thiele W, Wulf J. Extracranial stereotactic radiotherapy: Evaluation of PTV coverage and dose conformity. Z Med Phys 2002; 12: 221–9
- Wulf J, Haedinger U, Oppitz U, et al. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther Onkol 2001; 177: 645–55
- Wulf J, Meyer J, Flentje M, et al. Adaptive radiotherapy in stereotactic radiotherapy of extracranial targets: Integration of CT-verification on the treatment couch using a dedicated software to calculate the current stereotactic coordinate including evaluation of breathing mobility. Int J Radiat Oncol Biol Phys 2004; 60(Suppl 1)337
- Herfarth KK, Bahner ML, Lohr F, et al. Stereotactic single dose radiation therapy of liver metastases: Effects on normal liver tissue. Strahlenther Onkol 1998; 174(Suppl 71)76
- StatSoft. STATISTICA für Windows [Computer-Programm-Handbuch]. 6.0 ed. Tulsa, OK: StatSoft, Inc. 2300 East 14th street, Tulsa OK 74104; 1998.
- Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003; 124: 1946–55
- Wulf J, Baier K, Mueller G, et al. Dose-response in stereotactic irradiation of lung tumors. Radiother Oncol 2005; 77: 83–7
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradation for stage I Non-Small Cell Lung Carcinoma. Cancer 2004; 101: 1623–31
- Herfarth KK, Debus J. Stereotaktische Strahlentherapie von Lebermetastasen. Der Chirurg 2005; 76: 564–9