Abstract
Background: A focal reaction of the liver is radiologically seen after stereotactic high dose radiotherapy of liver tumors. The histological counterpart of this reaction should be clarified using an animal model.
Materials and Methods: Six New Zealand white rabbits were positioned on a special stereotactic set-up. Parts of the liver (1.5–8 ml) were irradiated with either 20–24 Gy/80% (n = 3) or 36 Gy/80% (n = 3). The animals were followed by CT examination up to 2 years after radiotherapy. Finally, the animals were sacrificed and the liver macroscopically and microscopically inspected. Results: No focal reaction could be observed in any liver at any time by CT examination. The liver macroscopically and microscopically showed no changes 6 months or 2 years after radiotherapy.
Conclusion: Up to a single dose of 36 Gy/80%, rabbits seem to show no focal tissue reaction after high dose radiation therapy of small parts of the liver.
Introduction
Radiation treatment of the liver can lead to radiation induced liver disease (RILD). RILD is clinically defined as weight gain, increased abdominal girth, ascites and a substantial rise in alkaline phosphatase Citation[1]. The incidence of RILD is dependent on the dose and the volume of the irradiated liver. Recent series showed that irradiation of small volumes of the liver with a single high dose or using a hypofractionated focused approach does not lead to RILD if enough liver volume can be spared from irradiation Citation[2–5]. However, a focal radiologic reaction can be observed in areas, which were irradiated with doses above 1×14 Gy single dose Citation[2] or 3×10 Gy Citation[3] in patients treated in stereotactic treatment protocols. The histological counterpart to these radiological tissue reactions is not exactly known. Knowlegde of the histopathological counterpart might help to define limits of volume and dose in the use of stereotactic radiation therapy of liver malignancies. An animal model using stereotactic high dose irradiation of parts of the liver in rabbits should clarify the histological patterns.
Materials and methods
Six New Zealand white rabbits received a stereotactic irradiation of parts of their liver. The initial weight was median 4.8 kg (range 3.9–5.8 kg). All animals were held under standardized conditions at the research animal facility at the German Cancer Research Center. For treatment and follow-up, the rabbits were anaesthetized by intramuscular injection of Xyloacin (4 mg/kg body weight) and Ketanest (50 mg/kg body weight). All rabbits received and intravenous entry in the lateral ear vein for fluid substitution, further anesthetic drug application and contrast medium injection. The animals received 100% oxygen during examinations and treatment.
All animal experiments were approved by the responsible governmental institution (Regierungspräsidium Karlsruhe AZ 35-918581/97/99).
Treatment Planning and Treatment
A special set-up system was developed for stereotactic irradiation of the rabbits (See ). The system contained of a wooden base plate which could be mounted to the regular stereotactic head system (Leibinger, Freiburg, Germany). The rabbits were positioned on the left body side and abdominal breathing was reduced by an abdominal belt.
Figure 1. Rabbit in stereotactic set-up up. Abdominal breathing is reduced by an abdominal compression.

A Siemens Somatom plus4 CT scanner was used for CT examinations. CT examinations always contained a unenhanced series, an enhanced series 30 seconds after the begin of contrast agent administration and a late study (3 minutes after contrast i.v.). Six to eight ml contrast agent (Ultravist 300, Schering, Berlin, Germany) were given by manual injection in approximate 10 seconds for the aquisition of contrast enhanced images. The CT was performed as a spiral CT with a slice thickness of 2 mm after reconstruction.
For treatment, the data of the enhanced series were immediately sent to a treatment planning station and the rabbit remained in the stereotactic set-up under anesthesia.
The treatment planning program STP Version 4.0 (Stryker- Leibinger, Freiburg, Germany) was used for stereotactic treatment planning. The liver was completely segmented and a target point was determined. The median volume of the liver was 59 mls (range: 45–100 mls). Treatment was performed by a coplanar arc radiation using a round collimator (8 mm, 11 mm, or 14 mm) on a 6 MV linear accelerator (Siemens; Germany). The volume that was covered by the prescription isodose (80%) ranged between 2% and 9% of the liver volume (median 4.7%). The absolute volume ranged between 1.5 and 8 ml (median 5 ml). The animals were divided in a “low dose” group (prescription dose of 24–28 Gy/80% isodose) and a “high dose” group (prescription gose of 36 Gy/80% isodose).
Follow-up
Follow-up examinations were performed 1 month, 2 months and 6 months after treatment for all animals. Three rabbits were also examined 3 months after radiotherapy (2 “high dose, 1 “low-dose”). One animal of each dosage group was also examined 1 year and 2 years after irradiation. Follow-up examination included a CT scan under the same conditions as the treatment planning.
After the last follow-up examination, the animals were sacrificed, the liver was removed and sliced. Based on the treatment planning documentation, the volume of irradiation was identified, macroscopically inspected and samples of the high-dose region (>80% isodose) and untreated liver regions (<10% of the applied dose) were collected in formalin and Carnoy fluid.
The liver samples were embedded in paraffin. Slices were microscopically examined by an experienced pathologist (HJG) after staining with Heamatoxylin and Eosin (HE-staining).
Results
All animals showed a normal behavior after irradiation. The initial weight remained stable (<10% changes) during the whole follow-up period.
CT data
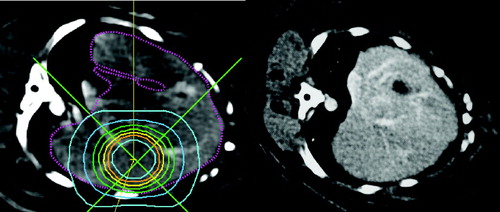
No changes of the high dose irradiated liver parts could be observed by CT examination. The parenchyma of the whole liver showed a homogenous density in the unenhanced, the enhanced and the late contrast enhanced CT studies in every follow up examination and in every dose group. The vessel architecture of the liver was clearly identified. (see ).
Figure 2. Treatment plan for a stereotactic liver irradiation (left): The inner circle corresponds to the 80% isodose (36 Gy), the following circles correspond to the 70%, 60%, 60%, 50%, 40%, 30% and 20% isodose lines. Right: CT examination 2 months after irradiation: no radiological reaction is visible.
Macroscopic and microscopic examination
No macroscopic and microscopic changes of the treated liver parts could be observed. Microscopically, liver of the high dose region did not show any changes of the normal portal liver architecture or congestion of the sinusoids.
Discussion
Recent publications showed that stereotactic radiotherapy of liver tumors can safely and successfully be performed Citation[3–5]. No radiation induced liver disease occurs if enough liver is spared from radiation. However, an asymptomatic focal liver reaction could be seen in all patients Citation[2], Citation[3].
This pattern of liver reaction is also described after fractionated radiotherapy Citation[6–8] and has been described in a patient showing radiation induced liver disease (RILD) after a single dose radiation treatment Citation[9]. Ingold et al. first described the clinical picture of RILD in 1965 Citation[10]. The mortality of RILD is approximately 10–20% Citation[1]. The pathophysiological counterpart is the picture of veno-occlusive disease (VOD). Reed and Cox were the first characterizing the histological changes of RILD as marked congestion, which involve mainly the central portion of each lobule with atrophy of the inner liver plates Citation[11]. The wall of the small veins reveal a large number of fine reticulin fibers that crisscross the lumen of the vein and the adjacent afferent sinusoids Citation[12]. Chronic radiation liver damage is characterized by a distortion of the liver architecture: variable distances between central veins and portal areas, fibrosis of the central veins and concentric fibrosis of portal spaces Citation[13]. It is not clear, if the focal reaction after stereotactic radiotherapy is a focal veno-occlusive disease. However, no focal tissue reaction could be observed by CT scanning in the rabbit animal model. Also, none of histological pattern of veno-occlusive disease could be detected in any of the irradiated animals.
In humans, this radiological reaction is first seen median 1.8 months after radiotherapy and decreases in volume thereafter Citation[2].
The radiological reaction occurs after a single dose greater than 14 Gy in the human liver. We applied up to 45 Gy to the isocenter of a 11 mm round collimator in a rabbit liver. Meaning, a volume of 20 ml received more than 14 Gy, 8,5 ml more than 20 Gy and 5,5 ml more than 30 Gy in a single fraction. However, we could not see any radiological tissue reaction. It is possible that normal tissues in rabbits are much less radiosensitive than in humans. On the other hand, there are several reports of successfully using rabbits to test radiation effects on other organs than the liver within the known dose limits Citation[14], Citation[15].
It is unlikely, that the reason of not detecting is based on a insufficient scanning technique. We examined the rabbits using a contrast enhanced CT with a contrast bolus injection resulting in a sharply contrasted images of the liver with clearly demarcated liver veins as it is usually seen in unaffected parts of human livers after radiotherapy. It might be possible, that we timely missed the focal reaction during our follow up examinations. We examined the liver 1 month, 2 months and 6 months after radiotherapy. The reaction showed up in human liver median after 1.8 months after radiotherapy with a broad range of 1.2 to 4.6 months. It decreases in size, thereafter, but is seen over a period of several months Citation[2]. If the reaction appears later in rabbits compared to the human liver or completely disappears earlier, we missed the correct time for the examination. On the other hand, 3 rabbits were also examined 3 months after radiotherapy without showing any tissue reaction on CT.
The irradiated volume might have been to small to see radiological changes. The volume that was covered by the prescription isodose ranged between 2 and 9% of the whole liver volume. However, the normal liver volume (including the tumor) that is covered by the prescription isodose is below 10% of the liver volume in humans in most cases and the radiological radiation reaction occurs at much lower dose levels.
The rabbits were moved within the complete stereotactic set-up from the CT scanner to the linac. The correctness of the target point was visually confirmed and no target point imaging were performed. Therefore, it cannot be guaranteed that we missed the target due to movement of the rabbit. On the other hand, due to the belly belt, the rabbits were fixed in their position and a large wheel pushing trolly enabled a smooth transport to the linac. Therefore it is unlikely, that the target significantly shifted. The abdomial compression belt should help to fix the rabbit and limit the liver movement. However, we do not know to which extent the moving was reduced, so the dose might be smeared around the target point.
Cromheecke et al. macroscopically described a change in tissue color 3 months after intraoperative irradiation of liver with electrons (up to 30 Gy) in dogs. Histologically, they described a thickening of the capsula and minor signs of veno-occlusive disease. The signs were more pronounced dependent on the dose. A significant focal fibrosis was visible 1 year after irradiation Citation[16], Citation[17]. No imaging studies were undertaken with these dogs. Other groups could not see any signs of veno occlusive disease in rats after doses of up to 40 Gy Citation[18–20]. Again, no imaging studies were done.
It remains speculative, what kind of pathological changes are responsible for the focal liver reaction after high dose stereotactic radiotherapy. It is most likely a focal veno-occlusive disease based on the case report by Willemart et al. Citation[9]
In conclusion, rabbits do not show a focal tissue reaction after high dose radiation therapy of small liver volumes. Therefore, the rabbit as used in this study is not a suitable animal model for examination of radiation effects in the liver.
We thank Mrs. Iris Moll for preparing and staining of the liver slides.
This work has been supported in part by the Medical Faculty Research Fund of the University of Heidelberg (Project number 124/1999)
References
- Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 1995; 31(5)1237–1248
- Herfarth KK, Hof H, Bahner ML, Lohr F, Höss A, van Kaick G, et al. Assessment of focal liver reaction by multiphasic CT after stereotactic single-dose radiotherapy of liver tumors. Int J Radiat Oncol Biol Phys 2003; 57(2)444–51
- Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A Phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 2005; 62(5)1371–8
- Blomgren H, Lax I, Göranson H, Kræpelien T, Nilsson B, Näslund I, et al. Radiosurgery for tumors in the body: clinical experience using a new method. J Radiosurgery 1998; 1(1)63–74
- Herfarth KK, Debus J, Lohr F, Bahner ML, Rhein B, Fritz P, et al. Stereotactic single dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol 2001; 19: 164–170
- Jeffrey RB, Jr, Moss AA, Quivey JM, Federle MP, Wara WM. CT of radiation-induced hepatic injury. AJR Am J Roentgenol 1980; 135(3)445–8
- Unger EC, Lee JK, Weyman PJ. CT and MR imaging of radiation hepatitis. J Comput Assist Tomogr 1987; 11(2)264–8
- Kolbenstvedt A, Kjøseth I, Klepp O, Kolmannskog F. Postirradiation changes of the liver by computed tomography. Radiology 1980; 135: 391
- Willemart S, Nicaise N, Struyven J, van Gansbeke D. Acute radiation-induced hepatic injury: evaluation by triphasic contrast enhanced helical CT. Br J Radiol 2000; 73(869)544–6
- Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation hepatitis. Am J Roentgenol 1965; 93: 200–208
- Reed GB, Cox AJ, jr. The human liver after radiation injury. A form of veno-occlusive disease. Am J Pathol 1966; 48: 597–611
- Fajardo L, Colby T. Pathogenesis of veno-occlusive disease after radiation. Arch Pathol Lab Med 1980; 104: 584–588
- Lewin K, Millis R. Human radiation hepatitis. A morphologic study with emphasis on the late changes. Arch Pathol 1973; 96: 21–26
- Tepe G, Dinkelborg LM, Brehme U, Muschick P, Noll B, Dietrich T, et al. Prophylaxis of restenosis with (186)re-labeled stents in a rabbit model. Circulation 2001; 104(4)480–5
- La Scala GC, O'Donovan DA, Yeung I, Darko J, Addison PD, Neligan PC, et al. Radiation-induced craniofacial bone growth inhibition: efficacy of cytoprotection following a fractionated dose regimen. Plast Reconstr Surg 2005; 115(7)1973–85
- Cromheecke M, Vermeij J, Grond AJK, Konings AW, Oldhoff J, Hoekstra HJ. Tissue tolerance of normal and surgically manipulated canine liver to intraoperative radiation therapy (IORT). Int J Radiat Oncol Biol Phys 1993; 27: 1141–1146
- Cromheecke M, Meijer D, Hietkamp J, Vermeij J, van Ginkel RJ, Hoekstra HJ. Beagle model used in a tissue tolerance study of the response of normal and surgically manipulated liver to single high-dose intraoperative radiotherapy. Lab Anim Sci 1996; 46: 640–647
- Bossola M, Merrick HW, Eltaki A, Bellantone R, Milligan AJ, Doglietto GB, et al. Rat liver tolerance for partial resection and intraoperative radiation therapy: regeneration is radiation dose dependent. J Surg Oncol 1990; 45: 196–200
- Geraci JP, Jackson KL, Mariano MS, Leitch JM. Hepatic injury after whole-liver irradiation in rats. Rad Res 1985; 101: 508–518
- Geraci JP, Mariano MS, Jackson KL. Hepatic radiation injury in the rat. Radiat Res 1991; 125(1)65–72
