Abstract
Surgical resection provides long term survival in approximately 30% of patients with colorectal carcinoma (CRC) liver metastases. However, only a limited number of patients with CRC-metastases are amendable for surgery. We have tested the effect of stereotactic body radiotherapy (SBRT) in the treatment of inoperable patients with CRC-metastases. Sixty-four patients with a total number of 141 CRC-metastases in the liver (n = 44), lung (n = 12), lymph nodes (n = 3), suprarenal gland (n = 1) or two organs (n = 4) were treated with SBRT with a central dose of 15 Gy×3 within 5–8 days. Median follow-up was 4.3 years. After 2 years, actuarial local control was 86% and 63% in tumor and patient based analysis, respectively. Nineteen percent were without local or distant progression after 2 years and overall survival was 67, 38, 22, 13, and 13% after 1, 2, 3, 4 and 5 years, respectively. One patient died due to hepatic failure, one patient was operated for a colonic perforation and two patients were conservatively treated for duodenal ulcerations. Beside these, only moderate toxicities such as nausea, diarrhoea and skin reactions were observed. SBRT in patients with inoperable CRC-metastases resulted in high probability of local control and promising survival rate. One toxic death and few severe reactions were observed. For the majority of patients, the treatment related toxicity was moderate.
Unlike most other cancers, colorectal carcinoma (CRC) often presents with solitary or oligo-metastases and most frequently they are located in the liver. This fact has let to an aggressive surgical approach in the treatment of patients with CRC metastases. Long term results from retrospective analysis of patients treated with resection of CRC liver metastases show 5-year overall survival rates of approximately 30% Citation[1], Citation[2]. Also patients with CRC lung metastases may benefit from surgical resection of metastases Citation[3], Citation[4]. Even though it is possible to perform extensive resection of the liver and the lung with removal of as much as 80% or 50% of the organs, respectively, the percentage of patients that are amendable for surgery is most often only in the range of 10–25%. There is therefore a great demand for other treatments for patients with CRC metastases. Non-surgical ablation methods such as cryotherapy, laser-induced interstitial thermotherapy (LITT) and radiofrequency ablation (RFA) with the latter being the most frequently used method, have been evaluated primarily in retrospective trials.
Stereotactic body radiotherapy (SBRT) is a non-invasive technique based on high-precision radiotherapy suitable for treatment of small targets in the body Citation[5], Citation[6]. The efficacy of SBRT has primarily been investigated in the treatment of limited stage small cell lung cancer Citation[7], Citation[8]. In the present study, SBRT was evaluated in patients with CRC metastases not amendable for surgery or other local treatment in a Danish joint prospective phase-II trial. The study was designed to focus primarily on tumor response, time to progression and toxicity.
Methods and materials
Patient selection
Patients entered the study from October 1999 to September 2003 based on the following criteria: Histological proven colorectal adenocarcinoma, radical resection of the primary tumor, inoperable judged by a trained hepato-biliary surgeon and not amendable for other local treatment, maximum diameter of largest metastasis 6 cm, and tumors visible on CT-scan. Only patients radically treated for the primary intestinal tumor and only patients who could be treated with SBRT in one session were included. Generally, one to four metastases was accepted, however patients with a higher number of metastases could be accepted if the expected relationship between dose and treated organ volume was tolerable. Patients should have WHO/ECOG performance status 0–2, had not received chemotherapy within 1 month before inclusion and had no symptoms possibly related to brain- or bone metastases. Primarily, patients with metastases in one organ were accepted, but in 2001 this was changed by an amendment so that also patients with metastases in two organs were accepted.
All patient were evaluated by contrast enhanced CT-scan of chest and abdomen prior to inclusion. Only five patients had a FDG-PET, and eight underwent laparoscopic ultrasound procedure before inclusion. The 51 patients treated in Aarhus were all evaluated by The Aarhus University Hospital Liver Tumor Board, which constitutes hepatobiliary surgeons, radiation and medical oncologists, medical hepatologists, and intervention radiologists.
The study was approved by the Ethics Committee of Aarhus County, informed consent was obtained from all patients and the study was carried out in accordance with the Helsinki Declaration II.
Radiotherapy
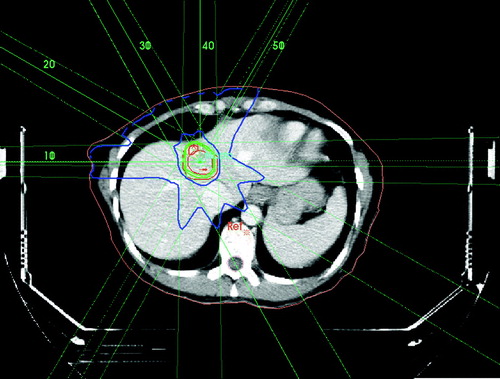
A detailed description of the principles of treatment has been given previously Citation[6], Citation[7], Citation[9]. Patients were firmly immobilized by custom made fixation systems that differed between the centres in Aarhus and Copenhagen. In Aarhus, patients were immobilised by the stereotactic body frame (SBF; Elekta AB, Stockholm, Sweden) with an external reference coordinate system visible on CT and a diaphragm control that reduces the internal respiration related movement of the liver Citation[6], Citation[7], Citation[9]. A maximum respiratory movement of the diaphragm of 10 mm during the respiratory cycle evaluated by fluoroscopy was allowed. In Copenhagen (n = 12), patients were immobilised by a custom made vacuum pillow and invasive skin marks. CT-scans were performed for treatment planning and in the 52 patients treated in Aarhus, an additional CT-scan was carried out to confirm the position of the isocenter at the day of first treatment. Spiral CT-scan was performed with 5 mm slice thickness (8 mm/s) reconstructed with 4 mm inter-slice distance. Intravenous contrast enhancement was used by injection of 125 ml Visipaque-275 with a flow rate of 4 ml/sec. and a delay of 70 s. Treatment planning was carried out in Helax (TMS) or CadPlan Plus/Eclipse, (Varian) (). The clinical target volume (CTV) defined as the visible hypodense tumor volume and eventually surrounding hypervascular rim was delineated by a radiotherapist and a radiologist in collaboration. A margin around the CTV of at least 5 mm in the transversal and 10 mm in the cranio-caudal direction was added to form the PTV. The CTV was encompassed by the 95% isodose, and the PTV by the 67% isodose surface. In Aarhus, the target isocenter was defined based on the SBF-coordinate system whereas in Copenhagen, the target isocenter was defined relative to a reference point defined by the vertebral spine. A total dose of 45 Gy prescribed to the isocenter was delivered in 3 fractions within an overall treatment time of 5–8 days. As a general principle, less than 30% of the liver received a total radiation dose of 10 Gy or higher and the spinal cord received a maximum dose of 18 Gy. Dose to the kidney, intestine and stomach was restricted to the lowest possible.
Treatment was delivered without respiratory gating on a Siemens Primus (Siemens) or a Varian Clinac 2100/2300 (Varian) most often by use of 5–8 static coplanar or non-coplanar 6–8 MV beams formed by multi-leaf collimator with leaf width of 5–10 mm at the isocenter. In Copenhagen, the position of the reference point was checked by portal imaging (PVI, Varian). In case of deviation, the position of the patient was adjusted and checked by a second portal imaging before treatment. All patients had prophylactic ondansetron 16 mg during the treatment period and in case part of the stomach or duodenum received significant radiation dose, the patients received pantoprazol for protection of gastritis or gastric ulceration for at least 28 days.
Follow-up
Evaluation of toxicity was performed at base-line, ½, 2, 3, 6, 9, 12, 18, and 24 months after treatment by use of the WHO performance status and toxicity grading system and CT-scans at base-line, 3, 6, 9, 12, 18 and 24 months after treatment. Any increase in grade from base-line was considered toxicity related to the treatment. According to the protocol, tumor response was evaluated on CT-scans using the WHO criteria. However, local control was later-on redefined as absence of radiological obvious or cytological proven recurrence. A local failure was defined as re-growth of tumor within or at the periphery of the irradiated volume. Other new lesions were considered distant failures.
Statistics
Local control and survival were evaluated by the Kaplan-Meier method and log rank statistics was used to test for differences in survival between groups. Pre-defined endpoints were tumor response, local control, progression (local or distant), survival and toxicity. Free from progression was defined as absence of any progression (local or distant). Survival data was updated in the Danish Central Personal Register at the time of analysis (2006/04/01). In all cases, a significance level of 5% was used.
Results
Patients
Sixty-five CRC-patients with a total number of 142 metastases were included in the study. One patient with an un-controlled local recurrent rectal cancer treated for a single liver metastasis was excluded at the time of analysis leaving 64 patients with 141 metastases for final analysis. Patient and tumor characteristics are given in and . Forty-four males and 20 females with a median age of 67 years (range 32–81) were included. Of these, 26 (41%) had rectal- and 38 (59%) colon cancer as the primary tumor with a median of 1.5 (range: 0–12.8) years before SBRT for metastases.
Figure 2. Distribution of 64 patients with CRC metastases according to age (A), number (B) and size of metastases (C).
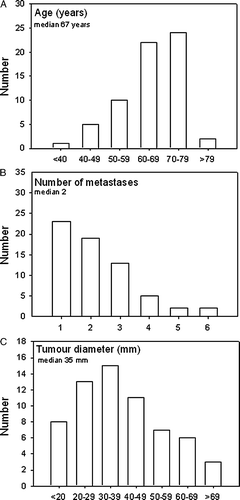
Table I. Patient characteristics of 64 patients included in the analysis.
Twenty-one (33%) of the patients had previously received local treatment for CRC-metastases, such as liver resection (n = 16), RFA (n = 3) and one patients had previous SBRT in Stockholm. Thirty-three (52%) patients had received neoadjuvant chemotherapy for metastasis before SBRT, 21 have had 5-fluorouracil and leuco-/isovorine, nine had CPT-11 alone or in combination and three had Xeloda. Median time from diagnosis of metastases to SBRT was 0.8 years (0–3.8 years). Of patients receiving neoadjuvant chemotherapy, 15 had a partial response, 11 had no change and seven had tumor progression.
All patients had WHO performance status 0–2. At baseline, four patients had grade 2 pain or higher due to metastases. The patients were all considered technical inoperable caused by number and localization of metastases (n = 53) or severe co-morbidity of the patient (n = 11). Patients were treated for a median of 2 (range 1–6) metastases and median diameter of the largest metastasis was 35 mm (range 10–88 mm) (). The majority of patients were treated for liver metastases (n = 44), whereas 12 were treated for lung metastases, three for lymph node metastases and one patient for a suprarenal gland metastasis. Four patients were treated for metastases in two organs, three of these for liver and lung metastases and one patient for lung and suprarenal gland metastases. Follow-up time was median 4.3 years (range 0.2–6.3 years). One patient was lost from follow-up 3 months after treatment. All other patients were followed within the study period defined from inclusion into the study until 2 years after treatment or progression or death.
Tumor response and local progression
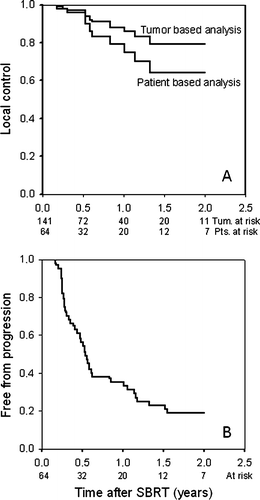
The patients were followed by CT-scans for 2 years. Within that time, local failure was observed in ten of 141 treated tumors. Only one patient had an isolated local failure without new distant metastases, whereas nine patients had simultaneous local and distant failures. In a tumor based analysis, this corresponds to a two year actuarial local control rate of 79%. However, since a large proportion of patients had more than one metastasis, the patient based local control rate was only 64% (A). Tumor progression occurred 3–16 months after SBRT. Patients with tumor diameter ≥35 mm did not have significantly increased risk of local recurrence compared with patients with smaller metastases. Treatment with neoadjuvant chemotherapy was not related with increased risk of local recurrence. At time of recurrence (local or distant), eight patients were retreated with SBRT, one patient underwent surgical resection and at least 26 patients received chemotherapy.
Progression free-, cancer specific and overall survival
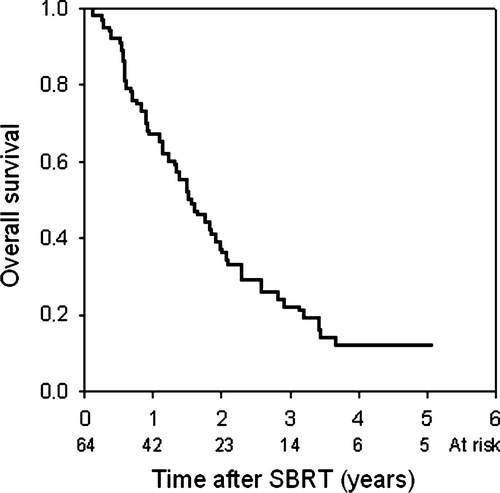
In 47 patients (73%), new lesions were observed during follow-up. The median time to progression was 6.5 months (95% C.I. 5.4–7.7 months) and the actuarial 2-year progression free survival was 19% (95% C.I.: 14–24%; B). In most patients, distant failures occurred in the same organ. The median cancer specific survival was 1.8 years (95% C.I. 1.4–2.3 years) and median survival 1.6 years (95% C.I. 1.2–2.0 years). Overall survival was 67, 38, 22, 13 and 13% at 1, 2, 3, 4 and 5 years after SBRT, respectively, when calculated from time of treatment (). If analysis was based on time from diagnosis of metastasis, the overall survival was 3.0 years (95% C.I 2.1–3.8 years).
Univariate analysis revealed that performance status <1 (p = 0.008), metachronous occurrence of metastases (p = 0.04) and absence of neoadjuvant chemotherapy (p = 0.01) were related with improved progression free survival, but only metachronous occurrence of metastases was significantly related with overall survival. Besides metachronous metastasis, female gender, extra-hepatic localization and largest metastasis less than 35 mm were significantly related with better overall survival ().
Table II. Toxicity in 61 patients within 6 months after SBRT.
Toxicity
Toxicity was evaluated in 61 patients. One patient died 7 weeks after SBRT caused by hepatic failure. Whether this fatal incidence was caused by radiation induced hepatitis or to thrombosis could not be established. In this patient, 60% of the liver received a dose higher than 10 Gy and the median dose to the liver was 14.4 Gy. One patient had perforation of a colonic ulceration demanding surgery and two patients with duodenal ulceration were treated conservatively. In all three cases, part of the intestine received a total dose of 30 Gy or higher.
summarizes the toxicity scores within 6 months after treatment. The most frequent side effect was abdominal pain and increased consumption of analgesics. Grade 2 or higher pain score was observed in 18 (28%) patients, most often for less than 3 months and grade 2 or higher analgesic score was seen in 16 (25%). Five patients deteriorated in WHO-performance status to grade 2 or higher. Ten patients had moderate nausea (≥ grade 2), four had moderate diarrhoea (≥ grade 2) and skin toxicity (≥ grade 2) was observed in four patients, two with ulceration of the skin. Progression of toxicity score to grade 2 or higher or to performance status 2 or higher was observed in 48% of the patients within the first 6 months after SBRT.
Table III. Prognostic factors related with overall survival for 64 patients included in the analysis.
Discussion
During the last two decades local treatment has become a routine treatment for CRC liver metastases. Surgical resection is the most frequently used method and is by most regarded the standard treatment for liver metastases. In large patient materials from the French Association of Surgeons Citation[1] and Memorial Sloan-Kettering Cancer Center Citation[2], 28–37% of the patients survived 5 years after resection of metastases. In both studies, patients with multiple poor prognostic factors such as age, narrow surgical margins, extra-hepatic disease, node-positive primary, short disease free interval between treatment for primary tumor and metastases, large number of metastases and large tumor size had a 5 year survival rate of 14%.
Resection of extra-hepatic CRC-metastases has also been shown to be beneficial. In a study of patients with extra-hepatic CRC-metastases of miscellaneous localizations, 32% of the patients survived after 5 years Citation[3] and in a study of CRC-lung metastases, 45% of the patients survived 5 years after resection Citation[4]. Even though these retrospective studies represent highly selected patients and that a large proportion of patient have received systemic treatments that affect the survival, these results are superior to what should be expected in patients with metastatic CRC Citation[10].
Promising survival rates in retrospective studies have justified that surgery is considered first choice of treatment for patients with CRC metastases. However, minimal invasive methods have been introduced in the treatment of patients with liver metastases from CRC with promising local control and survival rates. Most studies on the alternative methods are retrospective studies with short follow-up time including patients with primary as well as secondary liver tumors and the patients often receive a great variability of neoadjuvant and adjuvant systemic treatments. Furthermore, different ablation techniques have been used in the studies. Varying imaging techniques as guidance for the treatment have been used and some studies are based on intraoperative ablation whereas other studies used percutaneous technique. Cryotherapy was the first method introduced in the treatment of liver metastases as an alternative to surgery Citation[11]. Since then LITT Citation[12], Citation[13] and RFA Citation[14–16] were introduced and these are now the dominating methods for non-surgical treatment of liver metastases. Local control rates in individual tumor based retrospective analysis of patients with a variety of tumor types treated by LITT or RFA are in the range 54–98%.
A few studies report retrospective analysis on non-surgical local treatment of large patient cohorts with CRC liver metastases. In a study by Seifert et al. on cryotherapy where the majority of patients also received adjuvant hepatic artery chemotherapy, the 5-year survival was 13% Citation[17]. LITT was used by Vogl et al. in the treatment of 603 patients with 1 801 CRC liver metastases Citation[13]. In that study, 32% of the patients survived 5 years after treatment. In studies on RFA by Abdalla et al. Citation[18], Solbiati et al. Citation[19] and Berber et al. Citation[20] survival rates were 30–46% 3 years after treatment. Generally, large tumor size was the strongest prognostic parameter for recurrence or death, however, synchronous appearance, centrally located metastases and numbers of metastases had significant prognostic value in at least one of the studies.
In the present study on SBRT of CRC liver metastases with metastases primarily located in the liver and lungs, the local control rate at 2 years was 79% and survival rates were 22 and 13% at 3- and 5-years after treatment. In this study, SBRT was used for patients who were not amendable for surgery or RFA and thus represent a group of patients with a poor prognosis. Twenty-one (33%) patients had SBRT as second-line treatment after surgical resection or RFA of metastases. Furthermore, 33 (52%) patients received neoadjuvant chemotherapy for the purpose to downstage the disease before SBRT. These patients may have a poorer prognosis compared to patients that are treated at time of diagnosis. In the light of these facts, SBRT may be a potentially effective treatment.
In the present study, a number of factors such as WHO performance status ≥ 1, synchronous metastasis and treatment with neoadjuvant chemotherapy were related with short progression free survival. Overall survival was shortest for males, hepatic localization of metastases and for patients with tumors larger than 35 mm. Timing of metastasis and previous local treatment were of borderline significance. Most of these factors are well known prognostic parameters in treatment of CRC metastases Citation[1], Citation[2].
In a phase I/II from Heidelberg on single dose SBRT of liver tumors using increasing radiation doses of 14–26 Gy, local control after the higher doses reached 81% 18 months after treatment Citation[21]. In a study of SBRT of tumors in lung and liver performed in Würzburg, local control was 61% and survival was 43% 2 years after treatment Citation[22]. A variety of primary and secondary tumor types were included into the two German studies and therefore the survival rates cannot directly be compared to those of the present study. However, results of the present and the previous studies indicate equivalence in efficacy between SBRT and other non-surgical ablation techniques for treatment of CRC metastases and survival is comparable with results achieved after surgical resection of patients in poor risk groups in the large retrospective studies of the French Association of Surgeons Citation[1] and Memorial Sloan-Kettering Cancer Center Citation[2].
Complications after minimal invasive ablation of CRC-metastases are reported with great variability. After 219 cryotherapy sessions, one patient died (0.5%) and 27.6% of the patients experienced serious adverse event of various kinds Citation[17]. Vogl et al. reported three deaths and infrequent severe adverse events whereas the majority of patients only had minor complications in an analysis of a large series of 2 132 LITT-procedures in patients with primary or secondary liver tumors Citation[23]. In a study on RFA of 608 patients by Curley et al. Citation[24] there were three treatment related deaths (0.5%) and complications in 58 patients (9.5%). Severe events after these minimal invasive procedures generally includes abscess of the liver or peritoneum, bile duct injury, pleural effusion, segmental liver infarction or haemorrhage or haematoma that often requires surgical intervention or medical conditions such as cardiac or kidney failure, pneumonia or other infection.
In the present study on 64 patients with CRC metastases treated by SBRT, one death and three serious adverse events such as one radiation induced colonic and two duodenal ulcerations were recorded. In addition to these major events, 28% of the patients experienced abdominal pain most often lasting less than 3 months after SBRT and most of moderate intensity. In an American phase I trial using a 3-fraction schedule and total central doses of 36–60 Gy, no patients reached dose-limiting toxicity defined as grade 3 liver or intestinal or any grade 4 toxicity and only mild toxicity was observed in five of 18 patients Citation[25]. In the German phase I/II trial, only mild toxicity (grade 1 and 2) was observed with single central doses of 14–26 Gy Citation[21].
So far, little is known about the toxicity of radiation induced liver toxicity. By conventional fractionation, 25% of the liver can be irradiated safely with 28–32 Gy Citation[26]. In SBRT, hypodense regions of the liver occurring 1–3 months after treatment in volumes receiving 30 Gy in 3 fractions Citation[25] or 14 Gy in 1 fraction Citation[27] might be an indication of hepatic hypofunction. In SBRT of lung tumors, studies suggest that pneumonitis and lung fibrosis may be limited if small tumors are treated with central doses of 45–67 Gy in 3 fractions Citation[7], Citation[8], Citation[28].
SBRT is a non-invasive procedure that similarly to the other non-surgical methods allows local ablation of metastases. Whereas other non-surgical methods have almost exclusively been used for treatment of liver metastases, SBRT can be used equally well in lung and liver and until now it has primarily been used in the treatment of limited stage non-small cell lung cancer. By its precise and hypofractionated nature, it delivers a very potent dose to a small volume. Future improvements may even increase the efficacy of the treatment. Implementation of FDG-PET/CT in dose planning is supposed to improve the diagnostic accuracy significantly Citation[29]. PET/CT may image tumors that cannot be visualized by conventional contrast enhanced CT-scan and may give a more precise delineation of the tumor. In addition, image- and respiratory guided techniques increases the precision in delivery of the dose to the target Citation[30].
Unfortunately, there are very few randomized trials on local treatment on CRC metastases. So far, no randomized study has proved the efficacy of any of the local treatments. However, one study randomizing patients with CRC liver metastases between RFA and chemotherapy versus chemotherapy alone is currently accruing patients (EORTC-40004). Until results from prospective trials are available, non-surgical tumor ablation should be considered experimental and should mainly be performed within clinical trials.
In conclusion, the present study demonstrates promising local control for patients with CRC metastases primarily in the liver and lungs treated with SBRT. Survival of patients treated with SBRT was comparable with patients of the poor prognostic group treated with surgical resection and patients treated with minimal invasive techniques such as RFA, LITT and cryoablation. The only prognostic factor related with cancer specific survival was the size of the largest tumor. Re-treatment of new lesions was possible and in general, the toxicity of the treatment was moderate.
This study was supported by a grant from the Kloppenborg X-knife Foundation. The authors have no financial or personal relationships that inappropriately influence the presentation of the results of the present study.
References
- Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 1996; 77: 1254–62
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann Surg 1999; 230: 309–18
- Elias D, Liberale G, Vernerey D, Pocard M, Ducreux M, Boige V, et al. Hepatic and extrahepatic colorectal metastases: When resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol 2005; 12: 900–9
- Inoue M, Ohta M, Iuchi K, Matsumura A, Ideguchi K, Yasumitsu T, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 2004; 78: 238–44
- Blomgren H, Lax I, Naslund I, Svanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995; 34: 861–70
- Lax I, Blomgren H, Naslund I, Svanstrom R. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol 1994; 33: 677–83
- Hoyer M, Roed H, Hansen AT, Ohlhuis L, Petersen J, Nellemann H, et al Prospective study on stereotactic radiotherapy of limited stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2006; (in press).
- Timmerman R, Papiez L, McGarry R, Likes L, DesRosiers C, Frost S, et al. Extracranial stereotactic radioablation: Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003; 124: 1946–55
- Hoyer M, Roed H, Sengelov L, Traberg A, Ohlhuis L, Pedersen J, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol 2005; 76: 48–53
- Varadhachary GR, Hoff PM. Front-line therapy for advanced colorectal cancer: Emphasis on chemotherapy. Semin Oncol 2005; 32(6 Suppl 9)S40–S42
- Cooper IS, Stellar S. Cryogenic freezing of brain tumours for excision or destruction in situ. J Neurosurg 1963; 20: 921–30
- Vogl TJ, Straub R, Zangos S, Mack MG, Eichler K. MR-guided laser-induced thermotherapy (LITT) of liver tumours: Experimental and clinical data. Int J Hyperthermia 2004; 20: 713–24
- Vogl TJ, Straub R, Eichler K, Sollner O, Mack MG. Colorectal carcinoma metastases in liver: Laser-induced interstitial thermotherapy–local tumor control rate and survival data. Radiology 2004; 230: 450–8
- Wood TF, Rose DM, Chung M, Allegra DP, Foshag LJ, Bilchik AJ. Radiofrequency ablation of 231 unresectable hepatic tumors: Indications, limitations, and complications. Ann Surg Oncol 2000; 7: 593–600
- Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: Results in 123 patients. Ann Surg 1999; 230: 1–8
- Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA. Combined resection and radiofrequency ablation for advanced hepatic malignancies: Results in 172 patients. Ann Surg Oncol 2003; 10: 1059–69
- Seifert JK, Morris DL. Prognostic factors after cryotherapy for hepatic metastases from colorectal cancer. Ann Surg 1998; 228: 201–8
- Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004; 239: 818–25
- Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: Long-term results in 117 patients. Radiology 2001; 221: 159–66
- Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: A prospective study. J Clin Oncol 2005; 23: 1358–64
- Herfarth KK, Debus J, Wannenmacher M. Stereotactic radiation therapy of liver metastases: Update of the initial phase-I/II trial. Front Radiat Ther Oncol 2004; 38: 100–5
- Wulf J, Hadinger U, Oppitz U, Thiele W, Ness-Dourdoumas R, Flentje M. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther Onkol 2001; 177: 645–55
- Vogl TJ, Straub R, Eichler K, Woitaschek D, Mack MG. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: Experience with complications in 899 patients (2,520 lesions). Radiology 2002; 225: 367–77
- Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg 2004; 239: 450–8
- Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 2005; 62: 1371–8
- Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol 2005; 15: 279–83
- Herfarth KK, Hof H, Bahner ML, Lohr F, Hoss A, van Kaick G, et al. Assessment of focal liver reaction by multiphasic CT after stereotactic single-dose radiotherapy of liver tumors. Int J Radiat Oncol Biol Phys 2003; 57: 444–51
- Paludan M, Hansen AT, Petersen J, Grau C, Hoyer M. Can acute pulmonary morbidity following stereotactic body radiotherapy for non-small cell lung cancer be predicted by dose-volume histogram parameters? A prospective study of 28 patients. Acta Oncol 2006; (in press).
- Selzner M, Hany TF, Wildbrett P, McCormack L, Kadry Z, Clavien PA. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver?. Ann Surg 2004; 240: 1027–34
- Eccles C, Brock KK, Bissonnette JP, Hawkins M, Dawson LA. Reproducibility of liver position using active breathing coordinator for liver cancer radiotherapy. Int J Radiat Oncol Biol Phys 2006; 64: 751–9