Abstract
Stereotactic Body Radiation Therapy (SBRT) is a potent means of systemic cytoreductive therapy for selected patients with metastatic cancer. We here report an interim analysis of a prospective Phase I/II study of SBRT for liver metastases. Eligible patients with liver metastases met these criteria: (1) maximum tumor diameter < 6 cm; (2) ≤3 discrete lesions; (3) treatment planning confirmed ≥ 700 cm3 of normal liver receives ≤15Gy. The gross tumor volume (GTV) was expanded 5–10 mm to yield the planning target volume, which received 60 Gy in 3 fractions of SBRT over 3–14 days in the Phase II component of the trial. As of July, 2006, 36 patients have been enrolled: 18 in Phase I, 18 in Phase II. The median age was 58 years (range 27–91); the M:F ratio was 20:16. The most common primary sites were lung (n = 10), colorectal (n = 9), and breast (n = 4). Among 21 pts with ≥ 6 months post-SBRT follow-up (median 19 months, range 6–29), one instance of SBRT-related grade 3 toxicity occurred in subcutaneous tissue superficial to the liver. No grade IV toxicity occurred. For 28 discrete lesions treated (median GTV 14 cm3, range 1–98) the 18 month actuarial local control estimate is 93%. This interim analysis indicates that a very high rate of durable in-field tumor control can be safely achieved with SBRT to 1–3 liver lesions as administered in this protocol, to a prescription dose of 60 Gy in 3 fractions.
Stereotactic body radiation therapy (SBRT) is emerging as an expedient, safe, and potent means of physically targeted cancer therapy. In addition to applications in the primary treatment of selected early stage primary tumors, SBRT can also be considered for patients with good performance and a limited burden of systemic disease. In this setting SBRT functions as a systemic cytoreductive therapy, either eliminating or greatly reducing a patient's whole body disease burden Citation[1].
In a previously reported Phase I study of SBRT for liver metastases, it was established that an SBRT dose of 60 Gy in 3 fractions could be given to patients with 1–3 discrete liver metastases, as long as certain normal tissue dose constraints were respected Citation[2]. We hypothesized that a dose of that biological intensity should provide high levels of durable tumor control, and here we report an interim analysis of the continuation of that study as a Phase II trial, with particular attention to the primary endpoint of tumor control and secondary endpoint of late toxicity.
Materials and methods
Eligibility
The protocol was approved by the Institutional Review Board of all participating institutions. Male and female patients with liver metastases considered technically or medically inoperable were eligible, as were patients who declined surgery. The maximum individual tumor diameter had to be less than 6 cm, and no more than three discrete liver lesions could be present. Patients were required to have no evidence of progressive or untreated gross disease outside of the liver. Patients with any primary tumor histology were eligible except those with germ cell tumor, lymphoma, or leukemia. Additionally, the treatment planning procedure had to confirm compliance with the dose constraints listed in the following section (SBRT Planning and Delivery).
No prior radiation therapy to the targeted area in the upper abdomen was allowed. Patients had to have adequate pre-treatment baseline liver function, defined as total bilirubin < 3 mg/dl, albumin > 2.5 g/dl, and normal PT/PTT unless on anticoagulants. Serum levels of liver enzymes had to be less than three times upper limit of normal. Patients also had to have adequate renal function, defined as serum creatinine < 1.8 mg/dl or creatinine clearance ≥ 50 ml/min. Patients with a history of active connective tissue disorder were ineligible. Patients were not allowed to have chemotherapy within 14 days before SBRT, and no subsequent chemotherapy could be planned within two weeks of radiotherapy. Patients were ineligible if they had any active liver infection, including hepatitis C. The Karnofsky Performance Status had to be at least 70 (patient cares for self, though unable to carry on normal activity or do active work). The minimum age was 18 years, and all patients gave informed consent and signed a study-specific informed consent form.
SBRT planning and delivery
Details of the patient setup, simulation, treatment planning, and treatment delivery have been previously published Citation[2]. Briefly, patients were immobilized during computed tomography (CT) simulation and treatment using a body frame with reference fiducial markers or equivalent customized external vacuum-type or synthetic body mold. Respiratory control was achieved using facilitated breath-holding or abdominal compression. Planning CT images through the liver were often fused with pre-SBRT diagnostic CT images, magnetic resonance images (MRI) or PET-CT scan to aid in identification of the gross tumor volume (GTV) during treatment (). The GTV was considered to be identical to the clinical target volume (CTV) and was expanded by a minimum 5 mm radial margin and 10 mm cranio-caudal margin to create the planning target volume (PTV).
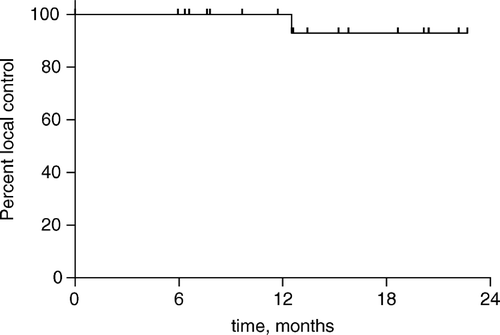
Figure 1. Actuarial in-field control after SBRT for 28 individual lesions in 21 evaluable patients with a minimum of 6 months follow-up.
SBRT was planned and administered using dynamic conformal arcs or multiple non-coplanar static beams generated by a linear accelerator with energies 6–15 MV. The dose was prescribed to the isodose line covering the PTV (generally 80–90% isodose line). Doses were calculated with tissue heterogeneity correction algorithms. Accurate patient repositioning and tumor relocalization were verified with at least one repeat verification CT scan of the patient in the treatment position I addition to daily orthogonal x-ray images or port films. Most subjects were given prophylactic anti-emetic therapy prior to daily SBRT. Daily total treatment times (number of minutes during which the patient was on the treatment table) were held to less than 45 min if possible in an effort to reduce any patient inconvenience or discomfort associated with extended immobilization and to lessen the chance that intra-fraction radiation repair would compromise the radiobiological intensity of treatment Citation[4], Citation[5]. The 3 fractions of SBRT administered to any individual lesion could be given on consecutive days or could be given with short breaks between fractions, according to institutional preference and patient-specific considerations; the total course of SBRT was to be given in no more than 14 days.
The prescription dose was delivered to the PTV. In the Phase I portion of the trial, this dose was escalated from 36 Gy in 3 fractions up to 60 Gy in 3 fractions without dose-limiting toxicity. Primary site or histology was not used to influence the dose prescription. In the phase II component, the prescription dose was 60 Gy in 3 fractions in all patients. indicates the distribution of patients evaluable for local control in terms of the study Phase in which they participated and the prescription dose given.
Table I. Characteristics of all patients evaluable for local control analysis (n = 21 patients with 28 treated lesions).
The normal liver dose constraint was developed in concordance with a “critical volume model” for normal tissue injury Citation[5]. Based on surgical reports suggesting that aggressive resections of the liver may be safely accomplished as long as at least roughly one fifth of the normal liver remains Citation[6], we applied a somewhat more conservative limitation by specifying that approximately one third of the liver should be spared from receiving a dose likely to cause notable dysfunction. Estimating that a typical normal liver volume is approximately 2000 cm3, we specified that a minimum volume of 700 cm3 should receive a total SBRT dose of less than 15 Gy. The basis for this dose level was a conversion from published experiences using conventional fractionation. The entire liver can safely tolerate at least 33 Gy in 22 fractions Citation[7], and the biologically equivalent dose of this schedule is 49.5 Gy-3, assuming a generic alpha-beta ratio of 3 and no significant repopulation effects Citation[8]. A dose of 15 Gy in 3 fractions would have a normal tissue BED of 40 Gy-3, conservatively less than the expected tissue tolerance.
Other normal tissue constraints applied: at least two thirds of the right kidney had to receive a total dose of less than 15 Gy, the percent of total kidney volume (defined as the sum of the left and right kidney volumes) receiving a total of 15 Gy had to be less than 35% of the total kidney volume, the maximum dose to any point within the spinal cord could not exceed 18 Gy total in 3 fractions (6 Gy per fraction), and the maximum point dose to the stomach could not exceed 30 Gy total in 3 fractions (10 Gy per fraction).
Statistics and endpoint analysis
The Phase II portion of the study was structured as a Simon 2-stage trial: in stage I a total of up to 13 patients will be enrolled and followed for 6 months before accruing additional patients. The primary endpoint for interim analysis was in-field local control at 6 months post-SBRT. The reason for specifying a minimum of 6 months follow-up to score local control was to avoid uncertainty associated with early transient radiographic changes in the surrounding liver parenchyma; patients who died without a minimum of 6 months post-SBRT imaging study follow-up are not considered evaluable for local control Citation[9].
The statistical analysis specified that a 6-month in-field control rate—here defined as local control of all treated lesions in an individual patient—of less than 60% will be considered too low (null hypothesis, H0: P0 = 0.6), and a control rate higher than 80% will be considered acceptable (alternative hypothesis, Ha: Pa = 0.80). If fewer than nine of 13 patients followed for a minimum of 6 months after SBRT maintain in-field control at six months post-SBRT, then the study would be discontinued. If at least nine patients in this initial group achieve local control of all treated lesions at the specified 6 month follow-up time point, then the trial advances to stage 2, where up to 22 additional patients will be accrued for a total of 35 evaluable patients in Phase II. If 25 or fewer patients, out of the total 35 treated patients attain control, then the alternative hypothesis will be rejected.
Post-SBRT liver imaging was obtained at 3 month intervals, and physical examinations and toxicity assessments were also performed at this interval. Actuarial local control curves were generated using statistical software that employs the product limit method of Kaplan and Meier (GraphPad Prism® version 4.00, GraphPad Software, San Diego, California, USA).
Results
Patient characteristics
As of June, 2006, a total of 36 patients have been enrolled: 18 in phase I, 18 in stage I of Phase II. The median age was 58 years (range 27–91); the male:female ratio was 20:16. The most common primary sites were lung (n = 10), colorectal (n = 9), and breast (n = 4). All patients were medically operable; in some cases resections of all lesions would have likely involved technical challenges in terms of proximity to major vessels and possible need for multi-lobar resection; however, no patient had attempted surgery that was aborted, so the true rate of unresectability based on technical concerns cannot be established. The median length of the treatment course was five days (range: 3–13 days). Five patients who died of progressive disease outside the treated field without a minimum of 6 months post-SBRT follow-up imaging are considered inevaluable for local control. In all five patients, initial post-SBRT imaging studies revealed characteristic normal tissue changes but no definite evidence of in-field tumor progression.
The treatment parameters of the 21 patients evaluable for local control and analyzed in this report are included in . Twelve patients from the phase I portion of the study are evaluable for local control after a minimum of 6 months follow-up. As of the date of this manuscript writing, nine patients under active surveillance who were enrolled in the first stage of the Phase II component of the study have had a minimum of 6 months follow-up, and all have maintained in-field local control. Thus, in accordance with the statistical analysis plan, the trial will continue to advance to the second stage of the Phase II component.
Local control
Among 21 patients evaluable with at least 6 months of post-SBRT radiographic follow-up (median follow-up 19 months, range: 6–29). For the 28 discrete lesions treated in these patients, the actuarial estimate of local control at 18 months is 93% (). The single patient who experienced in-field failure received a prescription dose of 54 Gy, and a sequence of pre-SBRT and post-SBRT images are presented in . The patient had stable radiographic changes at 12 months post-SBRT, and the treated lesion had diminished in volume from 47 to 9.5 cm3. A follow-up PET scan obtained 13 months after SBRT as a routine surveillance study revealed notable uptake in the treated lesion, and a subsequent CT scan at 15 months following SBRT revealed an increase in the volume of the liver lesion up to 15 cm3; the time of failure is scored as 13 months, the time of significant suspicion of recurrence confirmed on the subsequent CT scan.
Figure 2. Analysis of follow-up images for the patient with in-field recurrence. A, diagnostic images obtained, from baseline to 15 months post-SBRT. B, whole body PET scans, from time of failure until after salvage fractionated radiotherapy. C, dose distributions used in the salvage radiotherapy regimen, alongside fused image of CT-PET obtained before (left) and 3 months after (right) salvage radiotherapy. Note that the PET scan change appears to reflect a dose-response, with less activity inside the lateral aspect of the tumor volume that received higher dose.
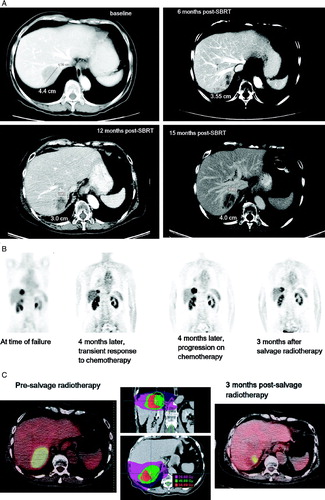
As it is also shown in , the patient proceed to have salvage chemotherapy (carboplatin and paclitaxel) and experienced transient response, as shown on follow-up PET scan obtained 4 months following the study that indicated failure. Unfortunately, the patient then experienced evidence of radiographic progression in the field again, seen on CT and PET. She was then offered salvage fractionated stereotactic radiotherapy, 40 Gy in 10 fractions, and demonstrated radiographic response on PET scan 3 months following this salvage regimen, with particular effect noted on follow-up PET imaging in the high dose region (C).
Toxicity
The normal liver tissue constraints were met for all treated patients. The median volume of uninvolved liver receiving less than 15 Gy total dose was 1 233 cm3 (range, 702–2 688 cm3). No radiation-induced liver disease was observed.
One patient with metastatic colon cancer had three liver lesions treated, each to 60 Gy, and after more than a year had intra-hepatic progression outside the treated regions as the only site of disease. The patient elected to undergo Selective Internal Radiation Therapy (SIRT) with Yttrium-90 micro-spheres (SIR-Spheres®, SirTex Medical, Inc., Lake Forest, IL, USA). Within weeks of having her left lobe treated she developed grade 3 gastritis, associated with weight loss and abdominal pain. She was treated with a proton pump inhibitor, H2 blocker and carafate for 9 months (continues presently) and recently had endoscopy showing pyloric stenosis that was dilated, resulting in improvement. Given that gastritis is a known toxicity of SIRT Citation[10] and the symptoms occurred soon after the SIRT, this event was considered unlikely to be related to the SBRT. For this study the patient was censored for both local control and toxicity follow-up on the date of first SIRT.
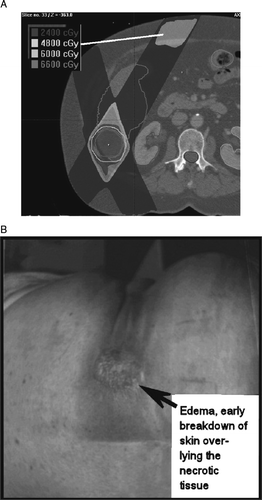
No instances of grade 4 toxicity occurred, but one instance of grade 3 soft tissue toxicity occurred. The patient was treated to a posterior right lobe lesion using five static non-coplanar beams. At the entrance point of one of the beams, skin redness and pain developed approximately 6 months after SBRT, worsening to the point of requiring narcotic analgesics. Eventually, there was subcutaneous tissue breakdown that reached the epidermis and required aggressive local intervention, including debridement and eventually a trial of hyperbaric oxygen (). According to the Common Terminology Criteria for Adverse Events v3.0 (CTCAE, ctep.cancer.gov/forms/CTCAEv3.pdf), grade 3 fibrosis in deep connective tissue is defined as “increased density with fixation of tissue; operative intervention indicated; interfering with activities of daily living”, and the toxicity in this case met these criteria. When the isodose distributions in this case were analyzed, it was noted that in the center of the involved area, approximately 1cm deep to the surface of the skin, there was a dose “hotspot” of more than 48 Gy corresponding to the region of injury.
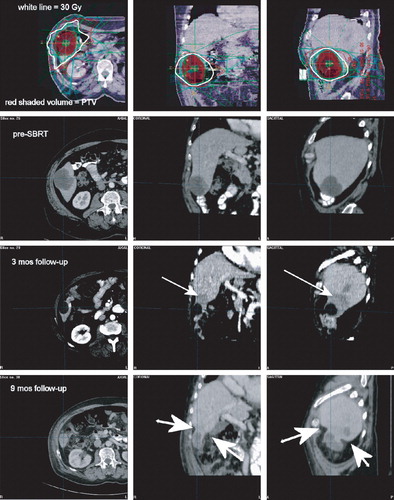
Figure 3. Normal liver atrophy following SBRT for a peripheral liver lesion. Uppermost panel, SBRT isodose distributions. Second panel, baseline pre-SBRT images. Third panel, 3 months post-SBRT; the arrow indicates the characteristic hypodensity observed at this time in surrounding uninvolved liver tissue. Bottom panel, 9 months post-SBRT; large arrows point to indentations in the liver contour representing atrophy of the normal tissue parenchyma.
Discussion
The interim analysis of this Phase I/II trial of SBRT for liver metastases indicates that a high rate of durable in-field tumor control can be safely achieved with SBRT to 1–3 liver lesions as administered in this protocol. The interim results support continuation of the trial into the second stage of the Phase II component, when additional patients will be accrued.
Treatment was generally well tolerated, but the observed case of grade 3 treatment-related soft tissue toxicity is instructive. The protocol did not specify a minimum number of beams or arc segments required for treatment, but it is very likely that this type of toxicity would have been avoided if more beams had been used, thus avoiding a “hotspot” deep to the skin. It is not possible to derive from this single event an estimate of the safe tolerance dose of subcutaneous tissue during SBRT treatment, but it is probably reasonable to consider a conversion of the safe linear-quadratic (LQ) model-based biologically equivalent dose (BED) from conventionally fractionated treatments in other sites. For instance, a dose of 66 Gy in 33 fractions, known to yield acceptably low soft tissue toxicity in other settings, would yield a BED of 110 Gy, assuming a generic alpha-beta ratio of 3 for late-responding normal tissues and ignoring time effects. By comparison a dose of 27 Gy in 3 fractions yields a BED range of 108 Gy, using the same alpha-beta ratio. It is our opinion that it is prudent to minimize the volume of extrahepatic normal tissue receiving doses in this range or higher, and with the use of multiple non-overlapping beams or arc segments, it is usually possible to accomplish this objective. Note that doses of 8–9 Gy per fraction are still likely within the range for which LQ model-based dose conversions are useful. The problems associated with use of the LQ model for higher doses are discussed below.
Non-toxic but possibly SBRT-specific normal liver tissue changes have been observed in several patients, and an illustrative case is shown in . Here, a comparison of pre-SBRT baseline images with images obtained later reveals apparent atrophy of surrounding normal liver parenchyma with extended follow-up. We and others have previously described the characteristic hypodensity that is observed in the first few months after liver SBRT Citation[2], Citation[9], but the reduction in normal liver volume observed here has not specifically been reported, to our knowledge. We interpret the findings to be supportive of the application of the critical volume model approach to normal liver dose constraints in SBRT in the sense that it should be considered that a certain volume of normal liver immediately surrounding the PTV might be ablated by the SBRT, hence the requirement that an adequate reserve is maintained for proper organ function. We suspect that the normal liver receiving low dose maintains regenerative capacity, but analysis of changes in liver volume over long follow-up intervals is beyond the scope of the present analysis.
Figure 4. Grade 3 soft tissue toxicity. A, SBRT isodose distribution; line points to area of injury, all within a subcutaneous volume that received more than 48 Gy. B, photograph obtained 8 months after SBRT.
Inter-study comparisons of clinical outcomes after SBRT are fraught with difficulties. Patient selection criteria are variable, dose prescription practices are potentially quite different at different institutions Citation[11], and post-SBRT assessments of local control might be influenced to some unknown extent by varying opinions among investigators, given lack of central review of images from different institutions. Furthermore, since different fractionation schemes are used, conversion to an index representing relative biological intensity can be difficult, given the fact that the applicability of LQ modeling to SBRT-range doses has been questioned Citation[12]. The major objection to LQ modeling for SBRT prescription doses is the possibility of intra-fractional repair that is not fully addressed by the LQ model. Nevertheless, despite those caveats, there can still be some value in considering from a broad perspective what parameters might be responsible for differences in clinical results.
To our knowledge there are two other published series in the literature in which a comparable regimen of SBRT was administered to patients with liver metastases and local control was reported as an actuarial estimate after 18 months of follow-up. In 2001 Wulf and colleagues from Würzburg reported an experience that included 24 tumor targets treated in the liver, 23 metastases and 1 cholangiocarcinoma. The GTV median and range was not reported, but the clinical target volume (CTV) was defined as the GTV + 2–3 mm margin, and the PTV was then created by adding 5 mm radially and 10 mm superiorly and inferiorly. All but one of the lesions received 30 Gy in 3 fractions, generally prescribed to the 65% isodose line. The median CTV was 50 cm3 (range, 9–516 cm3). The 18 month actuarial control rate was 61% Citation[13].
In 2005 Herfarth and Debus reported an update of a prospective Phase II trial with the inclusion of additional patients treated after closure of the initial trial. Seventy patients were treated with a single fraction of liver SBRT, sometimes termed liver radiosurgery Citation[14]. Details of tumor volume were not included in this report; median follow-up at the time of reporting was 14.9 months. After a dose of 22 Gy, the 18 month actuarial control rate was 66%. Site of origin was a prognostic factor in this series; patients with metastases from colorectal primaries experienced lower tumor control rates than others.
If the aforementioned caveats about the use of the LQ model for SBRT doses are ignored for the moment, the BEDs for the Wulf, Herfarth, and present series based on the typical prescription doses are 60, 70.4, and 180 Gy, respectively, assuming a standard tumor alpha-beta ratio of 10. Correlating these BEDs with the respective 18 month control rates of 61%, 66%, and 93% strongly suggests that a dose-response relationship exists and that the higher doses in the present series provided improved local control relative to the less biologically intense doses used in the other series. However, large prospective studies, with large numbers of patients with metastases of identical histology, would be required to understand with more certainty the true shape of the dose-control relationship for a variety of tumor types.
References
- Kavanagh BD, McGarry R, Timmerman RD. Stereotactic body radiation therapy for oligometastases. Semin Radiat Oncol 2006; 16: 77–84
- Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic bodyradiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 2005; 62: 1371–8
- Benedict SH, Lin PS, Zwicker RD, Huang DT, Schmidt-Ullrich RK. The biological effectiveness of intermittent irradiation as a function of overall treatment time: Development of correction factors for linac-based stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 1997; 37: 765–9
- Fowler JF, Welsh JS, Howard SP. Loss of biological effect in prolonged fraction delivery. Int J Radiat Oncol Biol Phys 2004; 59: 242–9
- Yeas RJ, Kalend A. Local stem cell depletion model for radiation myelitis. Int J Radiat Oncol Biol Phys 1988; 14: 1247–59
- Penna C, Nordlinger B. Colorectal metastasis (liver and lung). Surg Clin North America 2002; 82: 1075–90
- Russell AH, Clyde C, Wasserman TH, Turner SS, Rotman M. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: Results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys 1993; 27: 117–23
- Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy: A review. Brit J Radiol 1989; 62: 679–94
- Herfarth KK, Hof H, Bahner ML, Lohr F, Hoss A, van Kaick G, et al. Assessment of focal liver reaction by multiphasic CT after stereotactic single-dose radiotherapy of liver tumors. Int J Radiat Oncol Biol Phys 2003; 57: 444–51
- Andrews JC, Walker SC, Ackermann RJ, Cotton LA, Ensminger WD, Shapiro B. Hepatic radioembolization with yttrium-90 containing glass microspheres: Preliminary results and clinical follow-up. J Nucl Med 1994; 35: 1637–44
- Kavanagh BD, Timmerman RD, Benedict SH, et al. How should we describe the radiobiologic effect of extracranial stereotactic radiosurgery: Equivalent uniform dose or tumor control probability?. Med Phys 2003; 30: 321–4
- Guerrero M, Li XA. Extending the linear-quadratic model for large fraction doses pertinent to stereotactic radiotherapy. Phys Med Biol 2004; 49: 4825–35
- Wulf J, Hadinger U, Oppitz U, Thiele W, Ness-Dourdoumas R, Flentje M. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther Onkol 2001; 177: 645–55
- Herfarth KK, Debus J. Stereotactic radiation therapy for liver metastases. Chirurg 2005; 76: 564