Abstract
Cancer patients asked to participate in a randomised trial including chemotherapy at two university centres and a satellite centre were interviewed about perceptions and experiences (14 trial participating and 15 trial declining patients). The central phenomenon was a constant, cautious balancing of personal options searching for maximised effect, personal safety, trust, confidence and being cared for. Almost all developed a treatment preference and this was decisive for choices. Trial participants strongly wished to get the experimental treatment perceived as superior. They felt their freedom of choice being limited by randomisation. In contrast, trial decliners almost all focused on graver adverse effects related to the experimental treatment. A trusting and confident doctor-patient relationship was valued strongly. Yet, most breast cancer patients treated at the two large centres experienced a general lack of personal trust, confidence and being taken care of. The major reason was patients meeting too many physicians perceived as incompetent and unprepared. In contrast, the ovarian cancer patients treated at the satellite centre were content and satisfied with the main reason being the low number of physicians who were perceived as prepared, empathetic and knowledgeable. All patients expressed a feeling of “loneliness of autonomy” lacking sufficient knowledge and other resources to make educated choices.
The fraction of eligible patients recruited to clinical trials have decreased worldwide in recent years Citation[1], Citation[2], postponing trial completion and rendering the projective universality of trial results unpredictable Citation[3–5]. This development can only partly be explained by physician/system/societal factors Citation[3], Citation[5–13]. Patients expect and hope for interventions which can serve their personal needs as individuals, whereas clinical scientists in principle deal with patients as a group. This apparent collision of interests will supposedly be further aggravated by the increased trial management duties imposed by health service reforms including the EU directive effective by May 2004 on Good Clinical Practice (GCP) maybe decreasing future patient accrual as studies have indicated increasing recruitment problems with increasing complexity of logistics Citation[3], Citation[7], Citation[8]. Patient factors are, thus, probably gaining increasing importance Citation[5], Citation[9–15].
The knowledge about attitudes towards clinical research, reasons for accepting or refusing trial participation, patient perceptions of trial information, and how actual trial participation and treatment outside a trial setting after declining participation is experienced is limited. The majority of studies investigating attitudes toward clinical research have been based on hypothetical scenarios. They have in general revealed positive attitudes Citation[3], Citation[9], Citation[13–18]. Studies investigating reasons for acceptance of trial participation are relatively few, but in general both non-altruistic and altruistic motives are found to be important Citation[13–18]. These mostly questionnaire based studies have revealed valuable information, but research amongst patients with actual experiences of trial participation or treatment outside the trial setting is still rare Citation[17–19]. Consequently, it is almost unknown how it actually feels like to be the subject of clinical research. Furthermore, questionnaire studies only gain information about issues found relevant and interesting in advance, as even the best-prepared questionnaire cannot foresee the unexpected. A substantial risk of precluding detection of new and hitherto unnoticed significant aspects thus exists. In recent years, however, a growing number of studies using qualitative methodology have been published uncovering something of the psychological, emotional and social impact of taking part in a clinical trial from the perspective of the patient Citation[20–22]. The primary focus of qualitative research is how and why things work, what goes on, i.e. an attempt to understand the subtle meaning and significance of complex behaviour represented by the specific, individual, or distinctive, quality and character of phenomena relying on a few, carefully selected cases (strategic/theoretical sampling, case-studies), but many, sometimes infinite numbers of variables. The purpose of sampling is not, as in most quantitative studies, to establish a representative, random sample, but instead to seek out specific cases possessing characteristics relevant to the studied phenomenon.
Snowdon et al. using in-depth interviews with parents of critically ill babies randomised in a therapeutic trial, showed that the nature of the trial-and in particular the randomisation process-was poorly understood Citation[21]. Problems in understanding key aspects of a clinical trial have also been shown by others Citation[22–24]. Featherstone et al. interviewing trial participating and –declining men with lower urinary tract symptoms, found that both groups were engaging in a struggle to make sense of trial experiences, often developing alternative lay explanations of key trial concepts Citation[23], Citation[24]. Others report the invention of such lay explanations as well Citation[21], Citation[25]. Many subjects in these studies were expecting personalised care and treatment on the basis of individual therapeutic needs and developed clear treatment preferences having problems in accepting the random allocation of treatments. Several subjects mentioned the importance of trusting the doctor often extending this trust to the trial itself Citation[21–25].
The study reported here used in-depth qualitative research interviews amongst female cancer patients either accepting (trial participants) or declining (trial decliners) participation in one of three randomised clinical trials including chemotherapy. All interviewees had previously participated in questionnaire-studies Citation[18]. Our objective was to explore a broader description and understanding of the meanings assigned to patients’ lived experiences during their treatment courses within or outside a trial setting. This paper focuses on the findings with reference to the patients’ strategies in managing choices about trial participation and their decision-related experiences in a potentially life threatening situation. Other aspects of the study are reported in another paper Citation[26].
Materials and methods
We interviewed patients with either premenopausal breast cancer or advanced ovarian cancer following their decision whether to accept participation in a randomised clinical trial involving chemotherapy. The breast cancer patients were invited to participate in one of two randomised studies executed by the Danish Breast Cancer Cooperative Group (DBCG protocols 89b and 89d). The 89b-protocol compared chemotherapy (cyclophosphamide/floururacil/methotrexate (CMF), every 3rd week, 9 treatments in all) against ovarian radiation (given daily, 5 treatments). The 89d-protocol evaluated two chemotherapies (CMF versus a combination of cyclophosphamide/floururacil/epirubicin (CEF), both given every 3rd week, 9 treatments in all). Finally, the patients with ovarian cancer were randomised in a study comparing two chemotherapy-regimens (TC: paclitaxel/carboplatin; TEC: paclitaxel/epirubicin/carboplatin, both given every 3rd week, 6–9 treatments in all). In all three multicenter trials the experimental treatment was only available within the trial. The results of these trials (now completed) are still unpublished. Patients were interviewed after the completion of treatment. None of the authors – not being oncologists and not working with clinical oncology-were involved in any stage of the patients’ treatment or care.
The clinical studies were performed in Denmark at the Oncological Departments at Herlev University Hospital, Odense University Hospital, and Sønderborg Hospital during 1998–2001. Consent to be interviewed was obtained from patients who agreed to participate in a questionnaire-study exploring attitudes towards and experiences with clinical trials Citation[18]. The survey fulfilled the demands of Danish law and the Helsinki Declaration IV, and was approved by the relevant regional Research Ethics Committees (reg. no. KA 95059). During approval the Committee expressed ethical concerns about the interviewing of patients without a treatment response, and we were not allowed to conduct such interviews.
Sampling
All patients found eligible to participate in the clinical cancer trials were also eligible to participate in a questionnaire study, and only six of the approached patients in our full complex of questionnaire-studies declined to participate Citation[17], Citation[18]. Patients accepting to complete questionnaires were asked whether we could contact them at the end of the treatment to set up an interview. None declined this. After the completion of trial/treatment the potential interviewees were contacted by telephone, a new verbal consent was obtained, and an appointment was made to perform the interview. We initially planned to continue recruiting for the interviews until reaching a number of approximately 30 interviewees based on our experience from previous studies.
We chose to commence our initial interview-analysis including only women with breast cancer. During the early analysis we observed that the number and perceived experience of physicians at check-up appointments were important. This was already noted in preceding questionnaire studies also including cancer patients and non-cancer patients Citation[17], Citation[18], and we therefore decided to include also ovarian cancer patients from a questionnaire/interview study at the Oncological satellite centre at Sonderborg Hospital (unpublished) in the analysis. This small centre had only two oncologists attached in contrast to the university hospitals where a larger number of physicians treated the patients. The ovarian cancer patients declining participation in the clinical trial approached for interviews in Sonderborg during our sampling period unfortunately all had a lack of treatment response (4 patients). Thus, as our primary reason to include women with ovarian cancer was the low number of oncologists attached, we were forced to include only trial participating ovarian cancer patients for interviews. We thus used both consecutive and theoretical sampling methods.
The interviews
We used an interview-guide containing a semi structured list of topics. The audio taped interviews lasting 45–90 min were performed in the home of the patient. The interviews were transcribed verbatim by a professional agency (Akademisk Afskrivnings Anstalt, Copenhagen) and saved as single text files subsequently transferred to the computer-program The Ethnograph v5.07 (Qualis Research Associates, http://www.QualisResearch.com) for analysis.
Data analysis
The approach selected was the use of the version of the inductive constant comparative method described by Strauss & Corbin (Grounded theory) Citation[27]. The initial open text-coding involved examining each interview breaking the transcript down into individual units of meaning, and labelling them to identify categories, patterns and themes from the data. Next, evolving concepts were regrouped to form more abstract categories significant to the overall studied phenomenon. Categories were systematically sorted, compared, and contrasted yielding increasingly complex and inclusive themes until saturated. The nature of this analytic process was non-linear with the analyst turning back and forth between the different coding stages constantly reviewing memos and diagrams. The emerging themes were ultimately integrated and refined to identify a central theme able to link the majority of categories and form an explanatory whole. Finally, the findings were compared to the original tapes ensuring that untranscribed features (e.g. tone of voice, pauses etc.) did not contradict the transcribed text. Detailed records of the analytic process were kept in the form of extensive memo writing. All interviews were analysed together with the goal of developing the most comprehensive account of the data-set.
To maximise theoretical sensitivity and rigour all authors contributed to the analysis independently. SM fully analysed all interviews. SH performed a full open coding of five randomly chosen interviews. The codings were compared revealing an almost full agreement. The main differences in codings concerned the exact start or end of a given segment. Only a few new themes were identified and subsequently coded by SM. All authors participated in discussions through the analysis.
Illustrative quotations are given to make it possible for readers to judge interpretations. Pseudonyms are followed by patient characteristics including disease, status in trial (participant/decliner) and treatment allocation. Due to space limitations the surrounding context is not described in detail.
Results
Participants
Only patients with a treatment response were contacted (breast cancer: 9/11 trial participants; 18/21 trial decliners, ovarian cancer: 5/5 trial participants; 0/4 trial decliners). Three patients (all breast cancer trial decliners) contacted by telephone did not want to be interviewed when contacted. Twenty-four women with premenopausal breast cancer (9 trial participants and 15 trial decliners), and five patients with advanced ovarian cancer (trial participants) were interviewed. None had prior personal experiences with cancer trial participation, and only a few had experiences with non-cancer clinical research. Pseudonyms, ages, treatment received, and treatment centres are shown in .
Table I. Pseudonyms, -ages, and treatment allocation of interviewed subjects in randomised breast cancer trials (protocols DBCG 89b and DBCG 89d) and in a randomised trial in advanced ovarian cancer. Breast cancer patients: trial participants (median age 51 yrs); trial decliners (median age 49 yrs). Patients with advanced ovarian cancer: trial participants (median age 51 yrs).
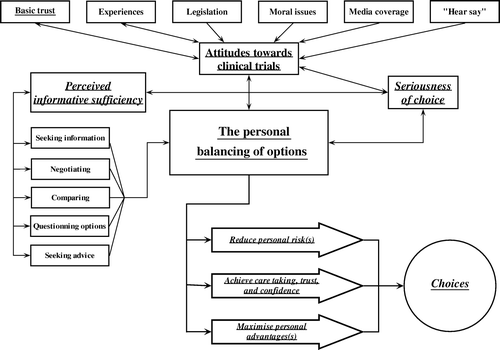
The core category – the personal balancing of options
During the early phases of analysis it was striking that all patients through almost every aspect of their experiences were doing the same thing, namely weighing pros against cons. This eventually proved to be the central phenomenon.
All patients had statements expressing an active, deliberate and careful balancing of personal options looking for information, weighing pros and cons, comparing and questioning options, seeking advice from near relatives and family doctors, and negotiating with physicians (). The goal was to maximise personal benefits, minimise risks and at the same time find confidence and trust. This strategy was expressed in many ways in all interviews not only concerning the choice whether to accept participation in the trial, but also concerning practically all aspects of the later treatment courses within or outside the trial-setting. The most important factors included in this balancing of options were (in random ordering) the feeling of personal safety, confidence and trust, the experienced seriousness of the situation/disease, the personal resources and feeling of illness, the presence/absence of perceived equipoise between treatments, and the attitude towards and experience of the randomisation process. A patient said:
“… What is this about, which consequences can there be, which side effects are there for me, where is my disease related to the risks I'd put my self in … You need to know what you are opposing. At least, I am … If I face that situation at another time, and I cannot see that it specifically is better for me to choose one from the other, then I would say YES … ” [Lone (breast cancer, trial decliner, ovarian radiation)]
Choosing in a potential life-threatening situation – a change of focus
Prior to their treatment none of the breast cancer patients had – apart from their postoperative course-felt ill, whereas the patients with ovarian cancer had had some illness preceding their diagnosis. However, practically all patients felt being in a life-threatened situation, and this aspect had significant – for some decisive-impact.
Many trial decliners argued that they probably would have participated in the trial if the choice had not had the character of choosing between life and death. Almost all experienced a radical change towards a more critical and hesitating position as the choice became a personal one. Nonetheless, they still maintained both a positive attitude toward clinical trials, and an acceptance of the necessity of trials Citation[26]. A patient stated:
“Generally speaking, I can see – using my common sense-the necessity of the trial, because that's the way you get things developed, get new knowledge and so forth, but in the situation, when it's yourself standing in it … It became entirely different for me … It was a shocking experience to realise that now it was personal and I couldn't participate. I was shocked that I couldn't contribute in helping others … I would very much have liked to do that, but I wasn't capable of it … [Ida (breast cancer, trial decliner, ovarian radiation)]
Chaos-feelings: choices in an emotionally turbulent state of mind
All patients were to different degrees feeling anxious, life-threatened and out of emotional balance. This state of mind had – for some significantly – influenced their capability of hearing and understanding the information they had been given. These feelings were expressed throughout the interviews in various ways. Janni (breast cancer, trial participant, ovarian radiation) said:
“I don't think you realise, that your life is in a chaos, when you get such a diagnosis. I couldn't-if it was high or low or what, I could in no way figure it out at all. I just thought that is was all over … You don't know if you have said YES or NO, and you don't know what it is you've accepted …”
The importance of trusting that doctors will treat you as a human being
The need for being taken care of, confidence, and reassurance was a feature of practically all interviews. The patients all sought reassurance that they were doing the right thing, and all – some desperately – needed someone to trust in this process.
A large majority had pronounced expectations and hopes of physicians primarily focusing on caring for them and treating them “as individual, human beings” including protection against risks. They all weighted this element of a trusting and confident doctor-patient relationship very strongly. Furthermore, a fundamental confidence in medical research keeping the patient “as an individual” in focus was put forward by most. Hanne (breast cancer, trial participant, CEF) said:
“Participating in a clinical trial is very much about trusting, that these people will not put me at unnecessary or irresponsibly risk … They will take care of me … if the confidence is broken it will be very dangerous to participate in trials …”
The majority of breast cancer patients did receive some individualised care taking from physicians. However, most patients related these experiences to single physicians, whom they got to trust, whereas generalised statements about doctors almost all expressed discontent and frustration due to the shifting panorama of seemingly inexperienced, unprepared and even uncaring physicians. The patients got the desired humane and caring contact from the nurses. The contact with the nursing staff was described as compassionate with nurses caring for the patients with a real, deeply felt interest for them as whole, independent individuals in a difficult and anxious life situation.
In contrast, the patients with ovarian cancer randomised at the satellite centre in Sonderborg were almost completely content and satisfied. They felt that they had been taken care of as individual human beings, and they strongly articulated confidence and trust in both physicians and nurses. They claimed that meeting a small number of physicians, who at the same time were perceived as empathetic and knowledgeable, was the main reason for this experience.
The loneliness of autonomy
An almost unanimous and strongly voiced experience amongst patients in all three groups was that their basis of knowledge was insufficient. They went through strong feelings of being lonely in the difficult and anxiety provoking situations faced during treatment courses with a life-threatening condition and a treatment resulting in – for some serious – adverse effects with large impact on their quality of life. Due to this loneliness, a large majority of patients expressed – some almost desperately – a need for confidence and trust.
There was, however, a profound gradient in this feeling between patient groups. The trial participants as a whole most clearly expressed the sentiment, and the breast cancer patients felt it the strongest. The patients all had to decide whether to participate in the trial; i.e. choose their own treatment (since even choosing randomisation was perceived as a form of choosing your own treatment course), although many would have preferred the doctor to make that choice for them. Still, the loneliness was not expressed only with reference to the choice whether to participate, but was articulated in relation to many aspects of the later treatment course. Statements from two patients exemplify:
“I couldn't decide – I wanted the doctor to decide which treatment was the best for me … I just remember that I chose my own treatment, and I didn't feel good about that at all … I was really surprised that they put me in that situation with such a serious disease, and want me to make choices about my own disease. It had an incredible effect on me, I felt bad about it, and I couldn't get the doctor to just give me an advice …” [Ida (breast cancer, trial decliner, ovarian radiation)]
“… but when you sit there and is in shock – I mean, its all wrong to start with a drawing of lots, because you really want to be in safe surroundings and be told that the treatment you're going to have will help you. And you don't feel that with a drawing of lots … I don't know anything about cancer treatment, and suddenly I had to decide a lot of things – it was a shock … that you choose yourself, its that – and I tried to say, that I did not know anything about it, and I need a proper explanation, but I didn't get that” [Susan (breast cancer, trial decliner, CMF + ovarian radiation)]
Perceived lack of clinical equipoise
The consensus state of uncertainty amongst clinicians at the start of a trial that no convincing justifications exists for supposing any patient advantaged or disadvantaged if allocated to one treatment arm rather than to another has been labelled clinical equipoise. An almost universal impression amongst patients was, however, that treatment options in the trials were not in genuine equipoise. As a result almost all patients developed a definite treatment preference.
Trial participants and decliners, however, in general considered different factors as deciding. The trial participants were largely concentrating upon perceived differences in treatment effect. A trial participant said:
“They sort of put forward that-the other was of course also good, but “the red chemo” – it was like they thought it maybe worthwhile to say YES to that, but I couldn't choose myself, they said…” [Gitte (breast cancer, trial participant, CEF)]
“… They asked me to participate in a treatment guaranteeing vomiting, loosing my hair and a lot more. You had to get a catheter implanted, and I already felt amputated – so, just very small surgical procedures made me feel all wrong. It was a thin-skinnedness and sensitivity which … it was like – I just couldn't have anything to do with it … It wasn't a great epic reflection, but – I already had had enough negativity and I wouldn't ask for more …”
“… I primarily didn't want to participate in this trial because I felt there was a health risk for me, and I didn't dare say YES … I was convinced that the new treatment was better … I have always been convinced that I would say YES to trial participation, but I was scared of this heart-problem and thought “I can't do this””
The freedom of choice with limitations
All patients clearly knew that they were free to either accept randomisation or to decline the offer and receive the standard treatment outside the trial setting. As described the large majority of trial participants got the impression that one treatment was more effective. They clearly considered this more important than differences in adverse effects, and this in combination with the strong wish to maximise personal chances of survival made their experience of freedom of choice a limited one. In fact, only two trial participants did not explicitly state a hope of “getting the new treatment”. Many patients directly articulated a feeling of being forced to participate in the trial, as this was their only way of “getting the new”. Inger (breast cancer, trial participant, CEF) exemplified this sentiment:
“… Yes, I would like to have the harsh one because I believed it better than the ordinary. I felt it bad about having to accept a drawing of lots, and what if I didn't win. I would have felt it as one in the eye, if I did not get it, because I really wanted it, but I was lucky to get it … participation in the trial gave me the chance of getting it … I felt awful about this limitation in the time before my knowing that I was in. The possibility of not getting it felt terrible …”
“… It would take good nerves to say YES to a drawing of lots, and then having to accept to be treated with the traditional treatment and at the same time thinking, that the new one is better … I would feel cheated, definitely. And not just cheated, but cheated on my life – we are out there, where we talk life and death – if you think the new one is much better than the known one …”
Randomisation
Almost all patients felt distressed by the “drawing of lots”. Yet, the majority accepted the principled necessity of the procedure, although many paradoxically did not understand the reason for it. At one extreme, Ida (breast cancer, trial decliner, ovarian radiation) very emotionally expressed being almost in a state of panic at the time of choice. She strongly wanted the doctor to choose for her and was totally unable to accept that a randomisation procedure should decide her treatment. For her this was decisive for her choice to decline trial participation. She chose her own treatment, but felt badly about it (see quotation of Ida in the paragraph The loneliness of autonomy). To illustrate more common degrees of disbelief see the quotations of Susan ((in the paragraph The loneliness of autonomy), Inger and Britt (both in the paragraph The freedom of choice with limitations). Hanne (breast cancer, trial participant, CEF) said when asked about her attitude towards randomisation:
“… the tossing of coins or drawing of lots is essential because the results of the trial could be misleading if certain types of patients were given certain treatments… I would have become very unhappy, if I hadn't had the new treatment … Drawing lots is necessary, but it seems – it is uncertain. When do we draw lots? We do that when we have a lottery or we gamble, I mean – when we take a risk, and that's not compatible with what doctors should stand for. They should stand for trustworthiness, safety, and “we take over” and “we are able”… the trusting relationship between doctor and patient suffers…”
In contrast, almost all trial participants (both patients with breast cancer and ovarian cancer) strongly wished to get the new supposedly most effective treatment. They strongly articulated discomfort and dissatisfaction with not just “getting it” and having to accept randomisation. Some of them even considered the procedure unethical, due to their clear impression of a lack of equipoise. Two trial participants said:
“… In that moment I thought it peculiar, that when you are going to have something, that it will be decided by a drawing of lots. They spoke highly about that chemo I got. However, they can just give it to me. Why must I be put in a pool, and then toss a coin about it? … I just think that the drawing of lots is strange. Why can't they just give you the one they think is the most effective? I did not understand … [Gitte (breast cancer, trial participant, CEF)]
“… I had a hope, that I had the new one in the draw … because I felt that I would be better off, having more of a chance. I felt that way … And after about 5 minutes they came back and told me, that I was not in … Yes, I got the two-drug treatment. At that time I felt: “why couldn't I choose myself?” … I remember, that I felt bad about that … I felt like having lost the drawing of lots …” [Anette (ovarian cancer, trial participant, TC)]
Discussion
The fundamental nature of patient experiences was the universal active, deliberate balancing of personal options with own best interests as the primary focus. Similar approaches toward solving trial-related problems have been found by others. Verheggen et al. demonstrated patients weighing up similar issues Citation[15], and Snowdon Citation[21], Featherstone Citation[23] and Cox Citation[22] showed their subjects engaging in analogous schemes to make sense of experiences during trial participation. This strategy probably reflects a basic human problem solving behaviour, which thus remained unchanged also in these patients’ anxious pursuits of hope, confidence and trust in a life-threatening situation. The patients’ personal estimate of the seriousness of the disease was an element of paramount importance. The graver the patients evaluated their situation the more they wanted to choose the right thing and consequently also the more negatively they assessed any factor imposing risk or uncertainty.
One of the most important ethical prerequisites for performing a clinical trial is the existence of clinical equipoise. It is in principle undisputed that lack of equipoise would render a trial unethical. However, not even specialists in the field are always in agreement concerning whether clinical equipoise exists, and the matter of acceptability of studies without equipoise and increasing differences in effects/adverse effects between treatment arms have been discussed Citation[28]. The experience or judgment of equipoise is, seemingly, a very personal matter depending on numerous factors. The result of this patient evaluation can very easily deviate from the physicians’ perceptions and there will be many cases where the patient cannot be persuaded of equipoise. If the patient experiences lack of equipoise, he/she will most likely decline trial participation unless the trial is the only chance of getting the treatment thought by the patient to be the best Citation[21]. It is surprising, however, that all patients here evaluated both possible effects and adverse effects, as we would have expected a focus primarily on the effect-side. A patient's choice was, however, mainly determined by whether the primary focus of the patient was on treatment effect or on adverse effects.
Acknowledging that almost all patients developed a treatment preference, it is understandable that the concept of randomisation was troublesome for most patients, and that some even considered it unethical. The expectations of personalised care with focus on individual therapeutic needs collide with the reality of the randomised trial. Appelbaum et al. named this the “therapeutic misconception” Citation[25]. A widespread unease with or problems in understanding randomisation procedures have been shown in other studies Citation[11], Citation[13], Citation[16–18], Citation[21], Citation[23], Citation[24].
The importance of a trusting and confident doctor-patient relationship as a part of any clinical encounter is undeniable, and in this interplay the physician's communication behaviours as perceived by the patient are of paramount importance Citation[8], Citation[10], Citation[11], Citation[29]. However, problems arise when doctors are also researchers Citation[6], Citation[12], Citation[25]. A significant finding was that a majority of patients felt being failed by the system/physicians they initially trusted. The patients felt life-threatened and lacked sufficient medical knowledge to make educated choices, but they did not get sufficient support from the physicians they met. They generally felt being left lonely in a state of emotional turbulence not knowing what to do.
It is noteworthy that trial participants expressed this loneliness most keenly. This is maybe to some extent explained by the fact that within choosing trial participation lay a larger risk than the risk taken by trial decliners who did not have to trust the physicians to the same degree. Still, this does not explain the gradient of “loneliness” between trial participants, as breast cancer participants clearly argued this stronger than the ovarian cancer participants. The main difference between the two groups of trial participants was that breast cancer patients all treated at the large university centres did not feel that they received the needed support, confidence and trust, whereas ovarian cancer participants all treated at a small satellite centre were content. All patients probably shared the same extent of inadequate medical knowledge from the start, but the combination of this and feeling deprived of support from physicians added consistently to the feeling of being “lonely in choices”. It is therefore not surprising that the trial participating breast cancer patients felt the loneliness of autonomy to the largest extent. A certain degree of this loneliness is probably inevitable. Yet, empathetic and understanding support and information from a few doctors, in whom the patient gains confidence, can apparently reduce the feeling of loneliness.
The two most important expectations from patients toward physicians and nurses are that they are competent, knowledgeable and prepared, and that they treat the patients as “whole human beings”. The importance of these factors has also been indicated in other studies Citation[14–19], Citation[22], Citation[25], Citation[29]. A part of the problem in seeing many doctors in a busy outpatient clinic is that some of them may be incompetent. Many of them will be unprepared in the sense that they – partly due to time limitations-do not know enough about what has happened earlier in the treatment course. Because of this they may be unable in relevant ways to “pick up the ball” from previous control visits. Experiencing being treated as “a whole person” implies not only feeling a positive emotional contact, but also that the patient does not sense physicians signalling an instrumental relationship to the patient. The patients often lacked knowledge about “hard” endpoints, and they instead put emphasis on their judgment of “soft” endpoints, where good relationships and impressions of whether they were listened to, taken care of, and taken seriously as individuals were the important issues.
The findings, as summarised above, may seem to be common sense. However, this study is one of the first to provide any evidence for a number of common sense beliefs such as the importance of contact with a limited number of health care professionals through the trial process, and the importance of salient side-effects for decisions to decline trial participation. It is well known from other areas of medicine that common sense is not always a reliable guide to knowledge and the findings in the present study are therefore of value for the evidence-based development of trial procedures.
To what extent can our findings be generalised? There are some important study limitations that must be considered before attempting to answer. We were not allowed to interview patients without a treatment response, and possibly our findings do not cover perceptions of patients with a poor outcome. We are fully aware that this fact limits the impact of our study-findings. We were, however, denied this option by the Danish regional ethical committee-system in spite of our arguments. It will in future studies be important also to include non-responders while obviously protecting them through an equally empathetic and sensitive approach as used when including treatment responding patients. In addition, interviews were carried out after the full completion of treatment courses lasting approximately 6 months. Therefore, it is impossible to know to what degree experiences of patients have changed impressions and views. However, our findings in the preceding questionnaire-study of a majority of respondents being relatively content, and of a general impression amongst respondents of an either very positive or positive trial experience Citation[18] have been challenged by the findings in the present interview-study and further stresses the importance of including patients at earlier time-points including pre-randomisation studies examining patients’ thinking also using a qualitative methodology Citation[22]. Finally, this study only included women. Whether male cancer patients would hold similar views is unknown, but the study by Featherstone et al. including only men-although not life-threatened cancer patients-showed similar strategies and views as those of these women with cancer disease Citation[23]. We attempted to improve rigour in our findings by several measures. The interviewed patients were part of a population already completing questionnaire studies examining similar topics. Further, we attempted to control codings by two authors coding part of the interviews, finding almost full agreement. Additionally, all authors were throughout the analysis actively participating in discussions, commenting on the analysis, making suggestions etc., and finally, it is important that findings ultimately were found consistent with the raw data. As a consequence, we find that our results are valid and reliable Citation[30]. Nevertheless, caution is needed when attempting to generalise the findings beyond current populations. Yet, the core category capturing the essence of patient experiences describes a universally applied problem-solving strategy likely to occur in similar forms in other contexts as well.
The authors wish to thank Poul Schlichting, Pia Munkholm from Medical Gastroenterological Department C, Copenhagen University Hospital at Herlev, and Bogi Davidsen, Department of Medicine, Slagelse County Hospital for general guidance. The greatest respect and thanks is addressed to the patients, who, regardless of their life-threatening disease and a difficult life-situation, were willing very openly to tell about and share their experiences. We are grateful to both physicians and nurses in the Oncological Departments at the hospitals in Herlev, Odense, and Sønderborg.
References
- Blichert-Toft, M, Mouridsen, H, West Andersen, K, the Danish Breast Cancer Cooperative Group (DBCG). Clinical trials. Sem Surg Oncol 1996;12:32–8.
- Klabunde CN, Springer BC, Butler B, White MS, Atkins J. Factors influencing enrolment in clinical trials for cancer treatment. South Med J 1999; 92: 1189–93
- Grunfeld E, Zitzelsberger L, Coristine M, Aspelund F. Barriers and facilitators to enrolment in cancer clinical trials: Qualitative study of the perspectives of clinical research associates. Cancer 2002; 95: 1577–83
- Gross CP, Mallory R, Heiat A, Krumholz HM. Reporting the recruitment process in clinical trials: Who are the patients and how did they get there?. Ann Intern Med 2002; 137: 10–6
- Lara PN, Jr, Higdon R, Lim N, Kwan K, Tanaka M, Lau DH, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrolment. J Clin Oncol 2001; 19: 1728–33
- Fallowfield L, Ratcliffe D, Souhami R. Clinicians’ attitudes to clinical trials of cancer therapy. Eur J Cancer 1997; 33: 2221–9
- Langley C, Gray S, Selley S, Bowie C, Price C. Clinicians’ attitudes to recruitment to randomised trials in cancer care: A qualitative study. J Health Serv Res Policy 2000; 5: 164–9
- Wright JR, Crooks D, Ellis PM, Mings D, Whelan TJ. Factors that influence the recruitment of patients to Phase III studies in oncology: The perspective of the clinical research associate. Cancer 2002; 95: 1584–91
- Comis RL, Miller JD, Aldige CR, Krebs L, Stoval E. Public attitudes toward participation in cancer clinical trials. J Clin Oncol 2003; 21: 830–5
- Grant CH, III, Cissna KN, Rosenfeld LB. Patients’ perceptions of physicians’ communication and outcomes of the accrual to trial process. Health Commun 2000; 12: 23–39
- Jenkins V, Leach L, Fallowfield L, Nicholls K, Newsham A. Describing randomisation: Patients’ and the public's preferences compared with clinicians' practice. Br J Cancer 2002; 87: 854–8
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: A systematic review. J Clin Epidemiol 1999; 52: 1143–56
- Solomon MJ, Pager CK, Young JM, Roberts R, Butow P. Patient entry into randomized controlled trials of colorectal cancer treatment: Factors influencing participation. Surgery 2003; 133: 608–13
- Slevin M, Mossman J, Bowling A, Leonard R, Steward W, Harper P, McIllmurray M, Thatcher N. Volunteers or victims: Patients views of randomised cancer clinical trials. Br J Cancer 1995; 71: 1270–4
- Verheggen FW, Nieman F, Jonkers R. Determinants of patient participation in clinical studies requiring informed consent: Why patients enter a clinical trial. Pat Educ Couns 1998; 35: 111–25
- Madsen SM, Holm S, Riis P. Ethical aspects of clinical trials: The attitudes of the public and out-patients. J Intern Med 1999; 245: 571–9
- Madsen SM, Holm S, Davidsen B, Munkholm P, Schlichting P, Riis P. Ethical aspects of clinical trials: The attitudes of participants in two non-cancer trials. J Intern Med 2000; 248: 461–73
- Madsen SM, Mirza MR, Holm S, Hilsted KL, Kampmann K, Riis P. Attitudes towards clinical research of participants and non-participants. J Intern Med 2002; 251: 156–68
- Verheggen FW, Nieman FH, Reerink E, Kok GJ. Patient satisfaction with clinical trial participation. Int J Qual Health Care 1998; 10: 319–30
- Pope C, Mays N. Reaching the parts other methods cannot reach: An introduction to qualitative methods in health and health services research. BMJ 1995; 311(6996)42–5
- Snowdon C, Garcia J, Elbourne D. Making sense of randomization; responses of parents of critically ill babies to random allocation of treatment in a clinical trial. Soc Sci Med 1997; 45: 1337–55
- Cox K. Assessing the quality of life of patients in phase I and II anti-cancer drug trials: Interviews versus questionnaires. Soc Sci Med 2003; 56: 921–34
- Featherstone K, Donovan JL. “Why don't they just tell me straight, why allocate it?” The struggle to make sense of participating in a randomised controlled trial. Soc Sci Med 2002; 55: 709–19
- Featherstone K, Donovan JL. Random allocation or allocation at random? Patients’ perspectives of participation in a randomised controlled trial. BMJ 1998; 317(7167)1177–80
- Appelbaum PS, Roth LH, Lidz C. The therapeutic misconception: Informed consent in psychiatric research. Int J Law Psychiatry 1982; 5: 319–29
- Madsen, SM, Holm, S, Riis, P. Attitudes towards clinical research amongst cancer trial participants and non-participants: An interview-study using a Grounded Theory approach. J Med Ethics. (in press).
- Strauss A, Corbin J. Basics of qualitative research. Techniques and procedures for developing Grounded Theory2nd ed. SAGE Publications Inc, Thousand Oaks, California 91320 1998
- Mackillop WJ, Palmer MJ, O'Sullivan B, Ward GK, Steele R, Dotsikas G. Clinical trials in cancer: The role of surrogate patients in defining what constitutes an ethically acceptable clinical experiment. Br J Cancer 1989; 59: 388–95
- Zachariae R, Pedersen CG, Jensen AB, Ehrnrooth E, Rossen PB, von der MH. Association of perceived physician communication style with patient satisfaction, distress, cancer-related self-efficacy, and perceived control over the disease. Br J Cancer 2003; 88: 658–65
- Barbour RS. Checklists for improving rigour in qualitative research: A case of the tail wagging the dog?. BMJ 2001; 322: 1115–7