Abstract
Stereotactic Radiotherapy has the potential to produce high local control rates with low risk of severe lung toxicity. From December 2000 to January 2006, 68 inoperable patients (median age 76 years) with stage I NSCLC received definitive hSRT. A mean total dose of 37.5 Gy (24–40 Gy; 60%-isodose) in 3–5 fractions was applied. Immobilisation was carried out by means of a vacuum couch and low pressure foil (Medical Intelligence, Schwab München, Germany). Staging procedures were thoracic and abdominal CT-scan, FDG-PET and CT or MRI of the brain in all patients. Clinical target volume was the tumor as seen in lung windowing of CT and in FDG-PET. Organ movements (6–22 mm) and patient positioning in the couch (3–12 mm) were added as safety margin for the definition of the planning target volume (PTV), that was enclosed by the 60%-isodose. We observed four (6%) local tumor recurrences, resulting in an actuarial local tumor control rate of 96%, 88% and 88% after 1, 2 and 3 year follow-up. Nineteen patients died, with eight patients due to cancer (12%), two to local tumor progression alone. Cancer-specific survival is 96%, 82% and 73% at 1, 2 and 3 years. Eleven patients died from comorbidities, making a 53% overall 3-year survival. Fifty five percent of the patients were affected by mild acute and subacute side effects, with only 3% experiencing pneumonitis III°. Late effects were pneumonitis III° in 1%, rib fractures in 3%, and benign pleural effusion in 2 patients. Hypofractionated SRT is safe even in elderly patients with stage I NSCLC and significantly reduced lung capacity. It leads to high local control rates and should be offered to patients not amenable for curative resection.
In stage I non-small-cell lung cancer (NSCLC) standard treatment is still surgery, in younger patients sometimes followed by systemic chemotherapy Citation[1], Citation[2]. The 5-year overall survival rates for pathological stage I range between 67% for pT1pN0 and 57% for pT2pN0 tumors. Based on clinical staging the corresponding values are 61% for cT1cN0 and 37% for cT2cN0, respectively. At 3 years, mean overall survival rates of about 70% in stage IA and of less than 50% in stage IB were published Citation[3–5].
Local tumor control is about 90% and depends on the type of resection. Lobectomy and pneumonectomy are superior to atypical resection Citation[4]. It is reported that the worse outcome with atypical resection is not only influenced by an increased local failure rate but mainly by perioperative morbidity and mortality. For these patients in early NSCLC stages with pre-existing comorbidity, advanced age or refusal of operation definitive RT alone may be the standard treatment option. Unfortunately, with conventionally fractionated and even moderately accelerated or hyperfractionated schedules the results are still less favourable than those obtained with surgery alone. The reported 5-year survival rates are as low as 18% (5–42) Citation[6].
High biologically effective radiation doses are generally of advantage with regard to tumor cell kill and local tumor control. Patients with clinically T1-2 N0-tumors seem to be the ideal candidates for investigation of newer technologies such as hypofractionated Stereotactic Radiotherapy (hSRT) Citation[7–9]. Initial data report on local control rates of up to 90%, with favourable results especially for patients in good general condition Citation[7]. The concept to irradiate the primary tumor (T1 or T2) alone is based on the well known observation that in these early T-stages the probability of involvement of locoregional lymph nodes is comparatively low Citation[10]. Nevertheless, it is suggested to perform the initial staging with FDG-PET to exclude mediastinal lymph node metastases. With a negative predictive value of about 98% a tumor infiltration can be excluded by FDG-PET and computed tomography with very high probability Citation[11], Citation[12].
Now, we present our current data on local tumor control, overall survival and early and late toxicity after hSRT in stage I non-small cell lung cancer. In all patients FDG-PET was applied as basic staging procedure and to define target volume, especially in tumor producing subsequent atelectasis. All patients had daily planning-CT in treatment position before each fraction.
Material and methods
Study design
This was a Phase I/II non-randomized trial. All patients underwent radiation treatment in curative intention. Baseline evaluations were performed before initiation of therapy: complete medical history; physical including cardiovascular examination and lung function test; determination of the ECOG performance status; full blood counts and biochemistry profile; thoracic and abdominal CT; MRI or CT of the brain. FDG-PET was used for the exclusion of mediastinal lymph node and distant metastases and to define the extension of the primary, especially in tumors producing atelectasis. Pleural effusion as well as thoracic wall or cardiac infiltration were excluded too. All patients underwent bronchoscopic biopsy and/or bronchioloaveolar lavage and/or fine-needle aspiration for histology or cytology before entering the study.
Eligibility criteria
All patients (≥18 years of age) had an ECOG performance status ≤2 and histologically or cytologically proven non-small-cell lung cancer stage I, without hilar, mediastinal or distant metastases. Patients were excluded if previous chemo- or thoracic radiotherapy had been administered. Operability had to be discussed and excluded by a multidisciplinary board including thoracic surgeons before initiation of hSRT.
Study endpoint
The primary end points of the study were clinical outcome defined according to the World Health Organization for response (local control defined as complete or partial remission as well as stable disease) as well as overall and cancer-specific survival. Acute and late toxicity were additional endpoints, mainly acute lung toxicity >CTC° I.
Radiotherapy
In the first eight patients, we used sequential CT-scans to define the accuracy and reproducibility of patient positioning in the commercially available vacuum couch (Medical Intelligence). CT-scans were superimposed by using the ExacTrak system (BrainLab) that produces errors of less than 1 mm using at least four markers. Reproducibility of the vacuum couch system was good, with mean deviations of 2.4 (0–5.5) mm in ventro-dorsal (y-axis) and 3.4 (1–5) mm in lateral direction (x-axis) only, but mean cranio-caudal deviation of 8 (2–15) mm (z-axis). The latter considerable error was a systematic one in cranial direction, likely caused by shrinkage of the vacuum couch within the first hours after preparation. Therefore, we introduced an interval of at least one day after preparation of couch before the planning CT-scan was carried out. By this, systematic errors could be avoided, and the random error was reduced to 3.5 (+/ − 1) mm in x, 1.5 (+/ − 1.0) mm in y-, and 1.5 (+/ − 1.0) mm in z-axis. Nevertheless, those deviations supported the evidence of the necessity of repeated CT-scan directly before each treatment session, to correct errors immediately before each fraction Citation[13].
Within the clinical protocol, radiotherapy was given as hSRT in curative intention, starting at 24.0 Gy total dose in 4 fractions. It was planned to escalate total dose to 30 Gy in 3 fractions Thereafter, the fractionation schedule and the single doses depended on lung function parameters, size and location of the target volume. For peripheral tumors a fractionation of 3 times 12.5 Gy was applied. For central tumors 5 times 7 Gy was the standard schedule. Dose was prescribed to the 60% isodose encompassing the planning target volume (PTV) Immobilisation was carried out by a vacuum couch and low pressure foil (Medical Intelligence) Citation[8].
Clinical target volume was the tumor seen in lung windowing of CT-scan. Information of FDG-PET was added mainly in patients with lung atelectasis. All areas suspicious in CT but with no increased FDG-uptake were excluded from the clinical target volume. Organ movements caused by breathing (lateral 0–6 mm, anterior-posterior 2–8 mm, cranio-caudal 6–22 mm) and reproducibility of patient positioning in the couch (3–12 mm) were determined for each individual patient by sequential CT- and orthogonal films before starting treatment. The individual values were taken into account as safety margin for the definition of the PTV. The PTV was enclosed by the 60%-isodose. Slice thickness on the CT-scan was 5 mm for three-dimensional treatment planning. Irradiation was applied with multiple coplanar static beams and/or dynamic arcs. Beam shaping was performed using an integrated motorized multileaf collimator with 1 cm leaf width. Calculation was done with the Siemens Helax system with pencil beam algorithm. Dose constraints for critical organs were set for spinal cord (max. 3 times 5 Gy or 5 times 4 Gy) and esophagus (max. 3 times 7 Gy or 5 times 5.5 Gy).
Toxicity and tumor control
During treatment all patients were interviewed and clinically examined for early radiation induced side effects on a daily basis. After hSRT, tumor response was evaluated by orthogonal radiographs and/or CT at 4–6 weeks together with clinical evaluation of side effects by a patient questionnaire and clinical examination as well as lung function and blood counts. At 8–10 weeks, and 4, 7 and 12 months, later in 6 months intervals, local control and adverse effects of radiotherapy were evaluated by CT (5 mm slice thickness), lung function test, blood counts, CRP, clinical examination, and patient questionnaire. In persisting tumors more than 18 months after hSRT or locally progressing lung tumor, FDG-PET-CT was done to exclude or confirm persistent cancer. In all patients with visible tumor in CT-scan but without evidence of uptake in FDG-PET the lesion was defined as scar and scored as complete remission. In cases with elevated uptake, further confirmation was attempted by bronchoscopy with lavage, brushing, and/or transbronchial biopsies. When persistent or recurrent tumor was confirmed, second line treatment was offered. Without confirmation of cancer, patients were followed in close intervals with repeated CT-scans and a second bronchoscopy after 8–12 weeks. If a patient developed a pleural effusion up to three repeated punctions within 12 weeks were performed to confirm or exclude malignancy.
Only tumors with local progression confirmed by histology or cytology, and/or new metastases were defined as recurrent or progressive, either local or distant. A complete response indicated that the tumor had completely disappeared or was replaced by fibrotic tissue, confirmed by a negative PET. A partial response was defined as a reduction of at least 50% in volume. Any suspicious residual density in CT-scan was considered to be a partial remission if FDG-PET was not negative. In summary tumor response was defined as follows:
complete remission = no sign of tumor in CT-scan and/or FDG-PET and/or bronchoscopy;
partial remission = reduction of at least 50% in tumor volume in CT-scan and/or reduced uptake in FDG-PET;
local progression = increase of tumor volume of more than 25% in volume in CT-scan and/or increased uptake in FDG-PET and positive histology in bronchoscopy;
distant progression = metastases in locoregional lymph nodes or in distant sites proven by CT-scan and/or FDG-PET.
Statistical analysis
Cumulative survival curves were calculated and drawn using Kaplan-Meier algorithms with the day of first treatment as the starting point. Actuarial local tumor control rates were determined using the Kaplan-Meier method assuming regrowth of the irradiated tumor or persistent uptake in FDG-PET as an event, irrespective of the status of other lesions outside of the treated volume. A tumor was censored in patients who died from other disease without tumor regrowth at that time and/or autopsy.
Results
From December 2000 to January 2006, 137 patients with malignant lung tumors were referred to our policlinic for Stereotactic Radiation. After evaluation of individual medical history and after completion of staging procedures (including FDG-PET) 68 patients were amenable for primary stereotactic radiotherapy alone in curative intention.
Of 68 patients, 48 were men and 20 women. Squamous cell carcinoma was the predominant type of cancer (n = 30). Nineteen patients had adenocarcinoma, 19 patients had non-small cell lung cancer not further specified. Mean age was 76 years (range 59–92). Follow-up is 17 months in mean (range 3–50). Mean planning target volume was 79 cm3 (16–299) (). Total dose was 37.5 Gy in mean (range 24–40 Gy), given in 3 to 5 fractions within 5 days (3–10 days) ().
Table I. Demographics and distribution of treatment parameters.
Table II. Dose escalation procedure (*individual decision).
Actuarial local tumor control at 1, 2 and 3 years is 96%, 88% and 88%, respectively (). In 26 patients second FDG-PET was carried out to confirm or exclude persistent or progressing malignant tumor. Follow-up imaging revealed a complete local response in 44 patients (64.7%), confirmed either by CT-scan alone or by CT-scan and FDG-PET. In 20 patients (29.4%) maximum response was defined as a partial remission. In four patients local progression occurred (5.9%). The patterns of first disease recurrence are listed in . Local, regional lymph node and distant recurrences were observed in 6%, 6%, and 16%, respectively.
Figure 1. Local recurrence-free survival (96% at 1 year, 88% at 2 years, 88% at 3 years), disease-specific survival (96% at 1 year, 82% at 2 years, 73% at 3 years), and overall survival (83% at 1 year, 71% at 2 years, 51% at 3 years) (according to Kaplan-Meier).
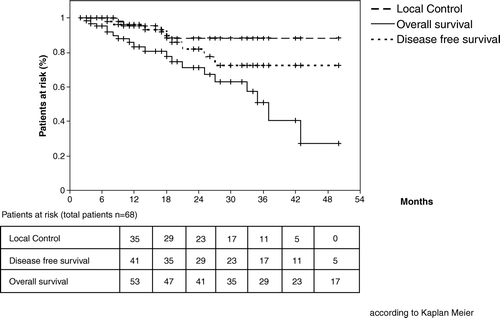
Table III. Distribution of first site of tumor recurrence and cause of death.
Disease-specific survival is 96%, 82% and 73% at 1, 2, and 3 year follow-up (). Eight patients died due to cancer, with only two of them by local tumor progression. No patient died due to radiation-induced toxicity, but 11 patients died due to severe comorbidity or a second cancer, resulting in an overall survival rate of 83%, 71%, and 51% at 1, 2, and 3 years follow-up, respectively ().
In general, severe toxicity was rare. Pneumonitis was the leading side effect. Fifty percent of our patients had signs of radiation-induced acute pneumonitis either clinically or radiologically (35.8 grade II, 12.8 grade 1). Clinical symptoms were apparent in maximal 39.1%, with only 6.4% grade 3 pneumonitis. Clinical symptoms and radiological signs in CT-scan both decreased during further follow-up. Only 19.2% of the patients had more than grade I lung sequelae later than 12 months after hSRT. Only one patient (2.8%) developed a grade III subacute pneumonitis 4 months after treatment. It persisted as a late pneumonitis and/or lung fibrosis for more than 2 years in this patient ().
Table IV. Lung toxicity (in percent) classified by clinical symptoms and radiological signs in CT-scan (*only clinical symptoms: maximum values).
Other side effects were fatigue (15% during or immediately after hSRT), shivering 5.1%, nausea 5.1%, and dermatitis 3.4%, with none being severe. Late effects were benign pleural effusion in 3.4%, rib fracture without confirmation of cancer in 5.0%, and a remarkable fibrosis of soft tissue of the thoracic wall in 3.4% of the patients (). In total, there was no obvious dose dependence regarding acute or late radiation induced side effects.
Table V. Non-lung toxicity (in percent/absolute values).
Discussion
Less than 25% of all patients diagnosed with lung cancer will present with early stage disease (less than 10% in stage I). These patients have the greatest hope for cure following standard procedure of resection. Survival vary, with reports on 5 year overall survival of 36% to 84% for pathologically proven stage IA and IB diseases Citation[4], Citation[14]. Mean values on overall survival at 5 years of 67% for postoperative pathological stage IA and of 57% in stage IB are reported, with a difference of 8–38% between stage IA and IB. The results decrease to 61% and 37% for preoperative clinically defined stage IA and IB, respectively Citation[3]. Mean 3-year overall survival rates of about 70% in stage IA and of less than 50% for stage IB are published for surgical treatment. These figures are comparable to our own data after hSRT alone.
Mediastinoscopy remains the gold standard for staging. However, FDG-PET is proven to be superior to CT-scanning alone, with 91% sensitivity and a 86% specificity for mediastinal disease and a negative predictive value of about 98% Citation[11], Citation[12]. The high accuracy of FDG-PET seems to be confirmed by our observation of a low regional failure rate during the first two years of follow-up. Only 6% of our patients developed an isolated initial tumor recurrence in hilar or mediastinal lymph nodes. These four patients could be treated in this area again, even when target volumes were overlapping, by radiotherapy or combined radiochemotherapy, resulting in a further regional tumor control for at least 12 months in all patients.
Unfortunately, data sets on overall survival with a longer follow-up after initial staging with FDG-PET-CT are still limited. This makes a direct comparison of our own data with results after curative resection as well as hSRT from other centers difficult Citation[15]. Considering disease-specific survival data one has to be aware of the fact that these are even scarcer than results concerning overall survival. Onishi et al. (2004) were able to demonstrate in a large multicenter trial, that overall survival after hSRT is comparatively better when patients are operable but refuse resection. In this subgroup of patients 3-year survival was significantly improved to 88% when a biological effective dose of more than 100 Gy was applied. These results are even better than those usually achieved by surgical procedures.
Most patients from our institution died from other causes than lung cancer, such as heart failure, second cancer, liver cirrhosis, exacerbation of severe chronic obstructive lung disease without signs of pneumonitis, etc. This explains the poor 3-year overall survival of 51% compared to 73% disease-specific survival and 88% local control rate at 3 years. We know from surgical data that even in patients with good general condition a difference of up to 20% between overall and disease-specific survival can be detected following resection, with a disease-specific survival of 72% for stage IA and of 32% for stage IB at 5 years Citation[5]. At 3 years for all stage I patients the disease specific survival was reported to be about 64%, which is even worse in comparison to our own data.
Comparable to surgical data, also in our patients the cancer relapse is usually a distant one. Only two patients died by a local recurrence (3%), but six (12%) due to distant metastases, predominately in brain and lung. This indicates that to a certain extent NSCLC is a systemic disease even in clinical stage I cancer patients. The use of additional systemic chemotherapy might be of benefit for selected patients after hSRT, such as those younger than 75 years. After resection the positive effect on survival has already been demonstrated in randomized clinical trials Citation[2].
We will continue to improve the results for our patients with stage I NSCLC treated with hSRT by optimizing the fractionation schedule and the total dose within a following single institutional trial. Additionally in future, to patients younger than 75 years we will offer systemic chemotherapy. Also in this clinical trial, FDG-PET will continue to be the central staging procedure.
We thank the “Bavarian State Ministry of Environment, Public Health and Consumer Protection” for generous financial support.
References
- Alam N, Shepherd FA, Winton T, Graham B, Johnson D, Livingston R, et al. Compliance with post-operative adjuvant chemotherapy in non-small cell lung cancer. An analysis of National Cancer Institute of Canada and intergroup trial JBR.10 and a review of the literature. Lung Cancer 2005; 47: 385–94
- Bernstein ED, Herbert SM, Hanna NH. Chemotherapy and radiotherapy in the treatment of resectable non-small-cell lung cancer. Ann Surg Oncol 2006; 13: 291–301
- Mountain CF. The international system for staging lung cancer. Sem Surg Oncol 2000; 18: 106–15
- Sugarbaker DJ, Strauss GM. Extent of surgery and survival in early lung carcinoma. Cancer 2000; 89: 2432–7
- Reed MF, Molloy M, Dalton EL, Howington JA. Survival after resection for lung cancer is the outcome that matters. Am J Surg 2004; 188: 598–602
- Zimmermann FB, Bamberg M, Molls M, Jeremic B. Radiation therapy alone in early stage non-small cell lung cancer. Sem Surg Oncol 2003; 21: 91–7
- Onishi H, Araki T, Shirato H. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: Clinical outcome in 245 patients in a Japanese multiinstitutional study. Cancer 2004; 101: 1623–31
- Zimmermann F, Geinitz H, Schill S, Grosu A, Schratzenstaller U, Molls M, et al. Stereotactic fractionated radiation therapy for stage I non small cell lung cancer. Initial results. Lung Cancer 2005; 48: 107–14
- Beitler JL, Badine EA, El-Sayah D, Makara D, Friscia P, Silverman P, et al. Stereotactic body radiation therapy for nonmetastastic lung cancer: An analysis of 75 patients treated over 5 years. Int J Radiat Oncol Biol Phys 2006; 65: 100–6
- De Ruysscher D, Wanders S, van Haren E, Hochstenbag M, Geeraedts W, Utama L, et al. Selective mediastinal node irradiation based on FDG-PET scan data in patients with non-small-cell lung cancer: A prospective clinical trial. Int J Radiat Oncol Biol Phys 2005; 62: 988–94
- Graeter TP, Hellwig D, Hoffmann K, Ukena D, Kirsch CM, Schafers HJ. Mediastinal lymph node staging in suspected lung cancer: Comparison of positron emission tomography with F-18-fluorodeoxyglucose and mediastinoscopy. Ann Thorac Surg 2003; 75: 231–5
- Grosu A-L, Piert M, Molls M. Experience of PET for target localisation in radiation oncology. Br J Radiol 2005; 78(S28)18–32
- Zimmermann F, Schill S, Geinitz H, Nieder C, Jeremic B, Molls M. Reproducibility of patient positioning in a vacuum couch for stereotactic radiotherapy (SRT) of NSCLC stage I. Strahlenther Onkol 2004; 180(Suppl 1)S15
- Manser R, Wright G, Hart D, Byrnes G, Cambell DA. Surgery for early stage non-small cell lung cancer. The Cochrane Database of Systematic Reviews 2005; 1.
- Hara R, Itami J, Kondo T, Aruga T, Uno T, Sasano N, et al. Clinical outcomes of single-fraction stereotactic radiation therapy of lung tumors. Cancer 2006; 106: 1347–52
