Abstract
In SBRT for NSCLC, highly potent radiation doses are delivered to patients with frequent pre-irradiatory compromise of pulmonary function. For the risk of pulmonary toxicity to be minimized during SBRT planning, data on its dose-volume dependency is needed. In the present study, we analyse the association of dose-volume histogram parameters with changes in dyspnea. The study concerns 28 medically inoperable stage I NSCLC patients that received SBRT at our department between 2000 and 2003. A central dose of 45 Gy/3 fractions was delivered in 5–8 days. WHO toxicity scoring of dyspnea was prospectively performed at baseline and during a 6-month follow-up post-SBRT. DVH parameters for pulmonary tissue were retrieved from the 3-D dose distributions.
Aggravated dyspnea was registered in 11 patients (40%). We found no association between DVH parameters and changes in dyspnea. Nor did we find any consistent temporal variations of dyspnea after SBRT. We identified COPD as the factor showing the closest association with aggravation of dyspnea. The observed aggravation of dyspnea following SBRT reflects habitual exacerbations of COPD rather than treatment-related toxicity. Concern about pulmonary toxicity should not be prohibitive for future studies targeting limitations to dose and volume.
Stereotactic body radiotherapy (SBRT) is acknowledged as a highly effective treatment for stage I non-small cell lung cancer (NSCLC). Tumor control rates of 80–90% after 1–2 years have been reported Citation[1–3]. Medically inoperable patients represent one third of all patients diagnosed with stage I NSCLC Citation[4]. The most frequent cause of medical inoperability is chronic obstructive pulmonary disease (COPD). Radiation-induced pulmonary toxicity may be more pronounced in patients with limited pulmonary reserve capacity. During treatment planning, the risk of pulmonary toxicity can be minimized if reliable dose-response data is available. While the correlation of dose-volume histogram (DVH) parameters with pulmonary toxicity is documented for conventionally fractionated radiotherapy (RT) Citation[5], Citation[6], similar data in the realm of SBRT is practically non-existent Citation[7].
The Department of Oncology at Aarhus University Hospital, Denmark, participates in a Danish phase II study of stereotactic RT for primary and metastatic extracranial tumors Citation[8], Citation[9]. The present paper concerns a subset of patients enrolled in this study, namely stage I NSCLC patients treated between 2000 and 2003. The objective of our study was to relate changes in dyspnea observed during a 6-month follow-up after SBRT to DVH parameters to give indications of the clinical pulmonary toxicity associated with our treatment regimen and of a potential dose-volume dependency.
Material and methods
Patient and tumor characteristics
Between January 2000 and September 2003, we enrolled 32 consecutive stage I NSCLC patients in the Danish phase II study of SBRT. For four patients we were unable to retrieve a complete DVH data set, so 28 patients were included in the present study. The NSCLC diagnosis was histologically verified except in one patient for whom verification by CT and PET was accepted. The patients were in WHO performance status ≤2. All patients were medically inoperable and had tumors with a non-central localization. COPD was the major or sole cause of medical inoperability in 21 of the 28 patients. Toxicity evaluation was performed at baseline and repeated at ½, 2, 3, and 6 months after completed SBRT. Median duration of follow-up within this timeline was 6.7 months (range 2.1–7.5 months). Details on baseline patient and tumor characteristics are listed in .
Table I. Baseline patient and tumor characteristics.
Treatment characteristics
Patients were immobilized in the Stereotactic Body Frame® (SBF) (Elekta AB, Sweden) for SBRT planning and delivery. Precise positioning of the patients was secured using a vacuum pillow and laser-guided skin marks. SBRT planning was carried out on a Helax-TMS treatment planning system and based on a planning spiral CT-scan performed after injection of intravenous contrast. The clinical target volume (CTV) was defined as the lung tumor plus surrounding spicular features and atelectasis. At least 5 mm transversal margins and 10 mm cranio-caudal margins were added to the CTV to define the PTV. Forty five Gy/3 fractions were delivered to the ICRU reference point within 5–8 days. The CTV was encompassed by the 95% and the PTV by the 67% isodose curve. SBRT was performed on a Siemens Primus or a Varian Clinac 2100/2300 by use of 5–8 static coplanar or non-coplanar beams shaped by multi-leaf collimators. An additional CT-scan was carried out in all patients to verify the position of the isocenter. All 28 patients received the 3 fractions prescribed.
Assessment of DVH parameters and dyspnea
We retrieved 3-D dose distributions and calculated DVHs for pulmonary tissue. We defined two volumes of interest (VOIs), the ipsilateral lung and both lungs defined as a single paired organ. In both cases, the VOI was delineated with exclusion of the CTV. The DVH parameters assessed for association with dyspnea were single-point parameters (Vdose: The percentage of pulmonary tissue receiving a specified radiation dose or above) and mean lung dose (MLD). The parameters were calculated by use of a pencil-beam algorithm and performed with tissue density inhomogeneity correction. Dyspnea was scored prospectively according to the WHO toxicity criteria. The grades can range from 0 to 4 to denote the following conditions: Normal/no change (0), mild symptoms (1), dyspnea on exertion (2), dyspnea at rest (3), and dyspnea confining the patient to bed (4). Aggravated dyspnea was defined as an increase in grade of dyspnea of ≥1 category above baseline at any time during the scheduled 6-month follow-up post-SBRT.
Statistical analysis
The SPSS package was used for statistical analysis of the association between DVH parameters and dyspnea. We also accounted for age, gender, performance status, cause of medical inoperability, tumor size, and tumor localization. The association of DVH parameters with changes in dyspnea was tested by use of Spearman's rho. When dichotomisation of variables was relevant, the Pearson χ2 test was used or Fisher's exact test when more appropriate due to expected low numbers. Two-sided tests were used and the level of statistical significance set at a p-value <0.05.
Ethics
The phase II trial was approved by the Committee on Biomedical Research Ethics of Aarhus County, Denmark. Informed consent was obtained from all patients, and the study was carried out in accordance with the Helsinki Declaration II.
Results
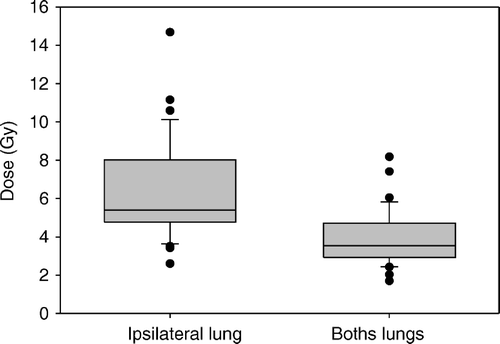
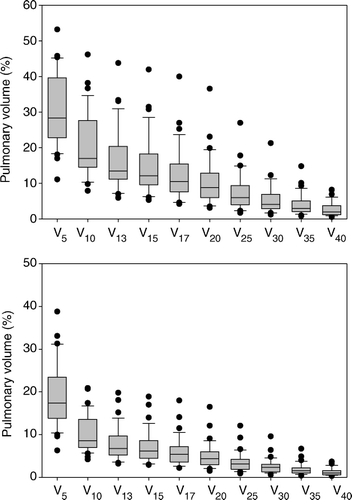
The radiation exposure of the ipsilateral lung mounted to a median physical MLD of 5.4 Gy (range 2.6–14.7 Gy). For both lungs considered a single paired organ, the median MLD was 3.6 Gy (range 1.7–8.2 Gy). A number of single-point parameters were calculated. In this study, V13 and V17 are assumed to correspond to V20BED and V30BED, respectively. This conversion between physical and biologically equivalent dosimetric parameters is based on the use of an α/β-ratio of 3.0 Gy. The DVH pertaining to the ipsilateral lung showed a median V13 of 13.5% (range 5.9–43.8%) and a median V17 of 10.5% (range 4.2–40.0%). For details on the radiation exposure of pulmonary tissue, see and .
Figure 2. Single-point parameters. Top: Parameters from the DVH for the ipsilateral lung. Bottom: Parameters from the DVH for both lungs.
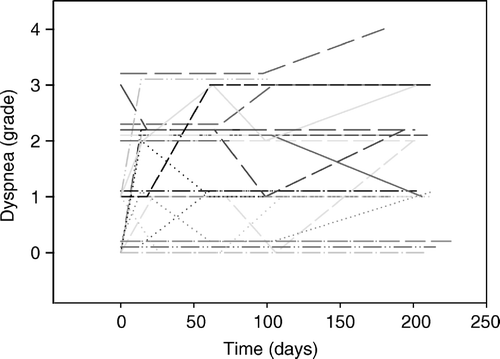
Baseline toxicity registrations showed that dyspnea was prevalent in 64% of the patient population prior to SBRT (). During follow-up, aggravation of dyspnea occurred in 11 of the 28 patients. In four patients an increase of one grade was observed while the remaining seven patients experienced an increase of two grades. The pattern was characterized by large inter-individual variability both regarding onset of aggravation and its duration (). Judged alone from the time line, aggravation of dyspnea showed no obvious relation to SBRT.
Univariate statistical analysis of the association of DVH parameters with changes in dyspnea showed no statistical significance. This was found for both single-point and MLD parameters () and for parameters derived both from the DVH for the ipsilateral lung and for both lungs considered a single paired organ.
Table II. The association of selected DVH parameters with aggravated dyspnea.
We addressed the association of aggravated dyspnea with age, gender, performance status, cause of medical inoperability, tumor volume, and tumor localization. No statistically significant associations were found (). COPD was the factor showing the closest association with the occurrence of aggravated dyspnea following SBRT. All seven patients experiencing an aggravation of dyspnea of ≥2 categories above baseline had a registration of COPD as the major or sole cause of medical inoperability. The dimensions of the study did not allow for meaningful multivariate analysis.
Table III. The association of patient- and tumor-related factors with dyspnea.
Discussion
Aggravation of dyspnea is a key feature of clinically apparent radiation pneumonitis (RP). Based on previous reports, the incidence of RP after SBRT for lung tumors is in the order of 4–20% Citation[1–3], Citation[10–18]. The occurrence of radiographic signs of RP is much more frequent. It can be demonstrated in 60–100% of the patients. A recent review on toxicity after SBRT for extracranial oligometastases summarizes the data from 15 lung studies Citation[19]. The authors find a low frequency of symptomatic RP and no clear indication of a definitive relationship between radiation dose and toxicity.
In our study, 40% of the patients experienced aggravation of dyspnea during a 6-month follow-up after SBRT. We found no association between irradiated volume of pulmonary tissue, radiation dose, and changes in dyspnea. In support of this finding was the time-course exhibiting a large inter-individual variation without any clear relation between the timing of SBRT and the occurrence of changes in dyspnea. Our prospectively documented aggravations of dyspnea might reflect habitual exacerbations of COPD rather than radiation-induced toxicity. No clinically relevant pulmonary toxicity seems to be induced by the SBRT regimen currently used in our department. A possible explanation to the findings could be the use of insufficient radiation doses. The toxicity associated with a treatment must, of course, be seen in the context of treatment efficacy. With a 2-year local control rate of 85% and a 2-year overall survival rate of 47%, the efficacy of our treatment regimen does not seem inferior to results reported from other studies on SBRT. This treatment efficacy is, however, the subject of another study Citation[9].
The phase II trial was not designed specifically for studies on pulmonary toxicity. Limitations to the present study are of course imposed by this fact. A future trial is underway that allows for a more complete exploration of SBRT-induced pulmonary tissue effects and their dose-volume dependency. We should study early and late effects, clinically apparent and subclinical, and local as well as global pulmonary toxicities. The design of this future trial will allow for this. As for now, the present study supports our notion of SBRT as a safe and effective treatment. Concern about pulmonary toxicity should not be prohibitive for future studies targeting current limitations to dose and volume.
This study was supported by the Danish Medical Research Council. The authors of this paper have no financial or personal relationships that inappropriately influence the presentation of the results of the present study.
References
- Hof H, Herfarth KK, Munter M, Hoess A, Motsch J, Wannenmacher M, et al. Stereotactic single-dose radiotherapy of stage I non-small-cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 2003; 56: 335–41
- Nagata Y, Takayama K, Matsuo Y, Norihisa Y, Mizowaki T, Sakamoto T, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005; 63: 1427–31
- Timmerman R, Papiez L, McGarry R, Likes L, DesRosiers C, Frost S, et al. Extracranial stereotactic radioablation: Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003; 124: 1946–55
- Nyman J, Johansson KA, Hulten U. Stereotactic hypofractionated radiotherapy for stage I non-small cell lung cancer–mature results for medically inoperable patients. Lung Cancer 2006; 51: 97–103
- Kwa SL, Lebesque JV, Theuws JC, Marks LB, Munley MT, Bentel G, et al. Radiation pneumonitis as a function of mean lung dose: An analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys 1998; 42: 1–9
- Rodrigues G, Lock M, D'Souza D, Yu E, Van Dyk J. Prediction of radiation pneumonitis by dose-volume histogram parameters in lung cancer–a systematic review. Radiother Oncol 2004; 71: 127–38
- Clenton SJ, Fisher PM, Conway J, Kirkbride P, Hatton MQ. The use of lung dose-volume histograms in predicting post-radiation pneumonitis after non-conventionally fractionated radiotherapy for thoracic carcinoma. Clin Oncol (R Coll Radiol) 2005; 17: 599–603
- Hoyer M, Roed H, Sengelov L, Traberg A, Ohlhuis L, Pedersen J, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol 2005; 76: 48–53
- Hoyer M, Roed H, Traberg HA, Ohlhuis L, Petersen J, Nelleman H, et al. Prospective study on stereotactic radiotherapy of limited stage non-small cell lung cancer. 2006; ( in press).
- Hiraoka M, Nagata Y. Stereotactic body radiation therapy for early-stage non-small-cell lung cancer: The Japanese experience. Int J Clin Oncol 2004; 9: 352–5
- Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: Clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004; 101: 1623–31
- Zimmermann FB, Geinitz H, Schill S, Grosu A, Schratzenstaller U, Molls M, et al. Stereotactic hypofractionated radiation therapy for stage I non-small cell lung cancer. Lung Cancer 2005; 48: 107–14
- Ohashi T, Takeda A, Shigematsu N, Kunieda E, Ishizaka A, Fukada J, et al. Differences in pulmonary function before vs. 1 year after hypofractionated stereotactic radiotherapy for small peripheral lung tumors. Int J Radiat Oncol Biol Phys 2005; 62: 1003–8
- Wulf J, Hadinger U, Oppitz U, Thiele W, Ness-Dourdoumas R, Flentje M. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther Onkol 2001; 177: 645–55
- Nagata Y, Negoro Y, Aoki T, Mizowaki T, Takayama K, Kokubo M, et al. Clinical outcomes of 3D conformal hypofractionated single high-dose radiotherapy for one or two lung tumors using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2002; 52: 1041–6
- Uematsu M, Shioda A, Suda A, Fukui T, Ozeki Y, Hama Y, et al. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: A 5-year experience. Int J Radiat Oncol Biol Phys 2001; 51: 666–70
- McGarry RC, Papiez L, Williams M, Whitford T, Timmerman RD. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: Phase I study. Int J Radiat Oncol Biol Phys 2005; 63: 1010–5
- Fukumoto S, Shirato H, Shimzu S, Ogura S, Onimaru R, Kitamura K, et al. Small-volume image-guided radiotherapy using hypofractionated, coplanar, and noncoplanar multiple fields for patients with inoperable Stage I nonsmall cell lung carcinomas. Cancer 2002; 95: 1546–53
- Carey SM, Katz A, Constine LS. Stereotactic body radiation therapy for extracranial oligometastases: Does the sword have a double edge?. Semin Radiat Oncol 2006; 16: 67–76