Abstract
Presentation of outcomes of patients treated by stereotactic body radiation therapy (SBRT) for lung lesions located within or touching a 2 cm zone around major airways. Serial tomotherapeutic SBRT has been planned and delivered at our institution since August 2001. Of 108 patients treated for primary and secondary lung tumors, nine harbored tumors (8 metastases, 1 recurrent NSCLC) located in close proximity to carina, right and left main bronchi, right and left upper lobe bronchi, intermedius, right middle lobe, lingular, or right and left lower lobe bronchi. SBRT was delivered to total doses of 36 Gy in 3 fractions (n = 8) or 6 fractions (n = 1), using a serial tomotherapy system (Nomos Peacock). We assessed local tumor control, clinical toxicity, normal tissue imaging changes, and overall survival. Median tumor volume was 26 cm3 (range 1.7 to 135 cm3). Tumor locations were hilar (n = 3), and parenchymal in six cases. Hilar lesions accounted for the three largest tumor volumes in the series. During a median follow-up of 10.6 months (range 2.5 to 41.5 months), all lesions treated were locally controlled as confirmed by CT or CT/PET imaging. Parenchymal imaging changes included focal lung fibrosis and major airway wall thickening. One occurrence of major airway occlusion (right lower lobe bronchus) was observed. This event was diagnosed by chest x-ray at 36 months, following treatment of the second largest hilar lesion in the present series. Based on the outcomes observed in this small sample series, SBRT for centrally located lung lesions appears feasible, was associated with low incidence of toxicities, and provided sustained local tumor control. However, long-term survival may be associated with major airway injury. As long-term follow-up in larger numbers of patients is lacking at this time, exclusion of patients with centrally located lesions may be considered when patients are treated in curative intent.
Stereotactic body radiation therapy (SBRT) delivered to primary and secondary lung tumors in single fraction of hypofractionated regimens has been associated with a favorable safety profile and encouraging local tumor control rates Citation[1–10]. However, observations of major airway toxicity when centrally located lesions were treated by SBRT have spurred concerns regarding the safety when addressing tumors in close proximity to carina, right and left main bronchi, right and left upper lobe bronchi, intermedius, right middle lobe, lingular, or right and left lower lobe bronchi Citation[3], Citation[11]. The presently accruing Radiation Therapy Oncology Group (RTOG) cooperative group trail RTOG 0236 subsequently stipulates exclusion of patients with tumors within 2 cm or touching a 2 cm zone around these major airways.
In a series of 108 patients treated by hypofractionated SBRT for primary or secondary lung tumors, we identified patients treated for centrally located tumors and assessed their outcomes with respect to local tumor control, treatment related toxicity, and overall survival. The present report aims to contribute to the relative paucity of data regarding major airway toxicity caused by SBRT.
Materials
Patient and tumor characteristics
Of 108 patients treated since August 2001 by SBRT for primary and secondary lung malignancies at The University of Texas Health Science Center at San Antonio/Cancer Therapy & Research Center, San Antonio, TX, USA, nine patients presented with centrally located lung tumors. Eight patients were treated for lung metastases; one patient was treated for recurrence of a primary non small-cell lung cancer. Lesion location was hilar in four cases (3 metastases, 1 NSCLC recurrence), and parenchymal in five cases, but with all of the lesion or aspects of it within 2 cm distance from either carina, right and left main bronchi, right and left upper lobe bronchi, intermedius bronchus, right middle lobe bronchus, lingular bronchus, or right and left lower lobe bronchi.
SBRT: Immobilization, simulation, and treatment concept
The details of SBRT treatment planning, and delivery are summarized briefly as they are reported in more detail in a companion paper submitted to this issue of Acta Oncologica (Fuss et al., Tomotherapeutic SBRT). All patients were immobilized using the BodyFix whole-body double-vacuum immobilization system (Medical Intelligence, Schwabmuenchen, Germany) according to published experience Citation[12], Citation[13]. Simulation imaging was acquired on a Picker/Marconi PQ5000 single slice CT simulator. All patients underwent a free breathing CT scan; PET scans were available for a subset of patients. Individually, either additional inhale/exhale CT scans or fluoroscopy through a simulator attached C-arm system was performed to assess respiration target motion. Image data were reconstructed in 3 mm slice thickness. The simulation image data were exported to an AcQsim virtual simulation platform for data co-registration with available positron-emission tomography (PET) scan data and then exported to a Corvus 5.0/6.0 treatment planning station (Nomos Corp, Cranberry Township, PA, USA). The target, defined as a gross tumor volume (GTV), was either based on CT image data or derived from both anatomical and metabolic image information. The motion envelope of a target volume was derived from co-registered inhale and exhale CT studies or approximated from fluoroscopy. For treatment planning, the GTV was expanded into a PTV by addition of 5 mm margins transaxially and 10 mm cranio-caudally as reported Citation[13], Citation[14].
Dose prescription was 3 times 12 Gy (6 times 6 Gy in one case; total dose 36 Gy) as the minimum dose to 95% of the PTV. Following typical dose prescription for radiosurgery planning with an emphasis on dose conformality and steep dose gradients, we aimed for an inhomogeneous dose distribution with maximum doses approaching or exceeding 150% Citation[15].
For SBRT delivery, all patients were immobilized in the BodyFix system on the simulator CT treatment table and underwent control CT imaging as reported Citation[6], Citation[13], Citation[15], Citation[16]. The acquired CT data were transferred to the Corvus treatment planning system for co-registration with the original planning CT study and assessment of patient and target setup Citation[13]. When a target setup to within 5 mm of planned position was assured, the patients were transferred in the BodyFix system onto the linear-accelerator treatment table and delivery was initiated.
Patient follow-up
All patients were scheduled for follow-up at 6 weeks and then in 3 months intervals. After one year of follow-up, these intervals were extended to between 4 and 6 months. Patients were examined clinically and typically underwent CT or combined CT/PET imaging for each follow-up. In addition, some patients underwent chest x-ray examinations. Objective lung function tests were not routinely performed.
Analysis
Data collected for analysis include tumor volumes, target location, clinical and imaging follow-up data as well as overall survival. Owing to the small sample size, only descriptive analysis (average, median, minimum, and maximum) was performed.
To establish length of follow-up, the date of last available patient contact or the date of death was considered. For assessment of local tumor control, and normal tissue imaging changes, the date of the last available imaging study or the date of the first study to demonstrate a change was considered.
Results
Volumes of targets
The median GTV volume was 26 cm3 (range 1.7 to 135 cm3), with corresponding PTV volumes of 12 to 211 cm3 (median 114 cm3), respectively. Hilar lesions were larger than parenchymal lesions with median volumes of 76.4 cm3 (range 44.7 to 135 cm3) versus median volumes of 13.5 cm3 (range 1.7 to 40.9 cm3), respectively.
Follow-up
Median follow-up was 10.6 months with a range from 2.5 months to 42.5 months. At time of analysis (June 2006), three of nine patients are alive with documented follow-up of 10.6, 14, and 38 months. Six patients have expired with a median time to death of 6.9 months (range 2.5 to 41.5 months). All patients expired from systemic disease progression. The latest available imaging follow-up in these patients confirmed local tumor control in six of six cases. All tumors treated in patients alive are locally controlled as of their last available imaging follow-up.
Imaging changes observed were partial tumor response (shrinkage >25%) or complete response in all instances (). When PET follow-up was available, uptake levels of less than 3, the threshold considered for response assessment, were achieved (data not shown). Lung parenchymal changes included three cases of transient pneumonitis (one requiring a short course of steroids); all follow-up imaging revealed fibrotic reactions at the site of lesions treated in CT studies acquired at 6 months or later ().
Figure 1. Central colorectal cancer metastasis; solitary remaining manifestation following resection of left sided colorectal cancer metastasis. Prescribed dose was 36 Gy (displayed is the 100% isodose in blue, 90% in red, 70% in yellow, and 50% in green). Two follow-up CTs at 8 and 27 months document complete tumor response and focal fibrosis. The patient expired at 41 months without evidence of clinically relevant lung toxicity of systemic progressive disease. This patient underwent two additional SBRT courses for new lung metastases.
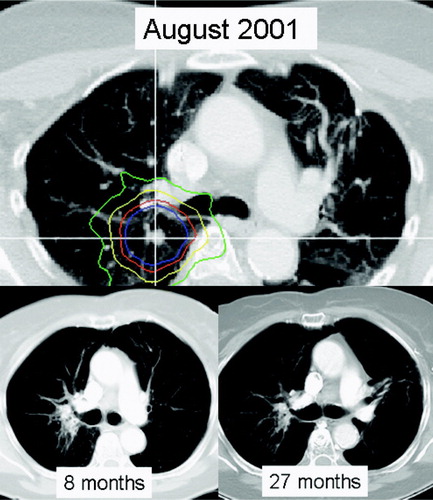
Figure 2. Solitary central malignant melanoma metastasis. Prescribed dose was 36 Gy (displayed is the 100% isodose in blue, 90% in red, 70% in yellow, and 50% in green). Two follow-up CTs at 16 and 36 months document focal fibrosis and complete tumor response. The patient expired at 38 months without evidence of clinically relevant lung toxicity. Despite new systemic disease manifestations in bones and brain, no additional lung metastases were diagnosed.
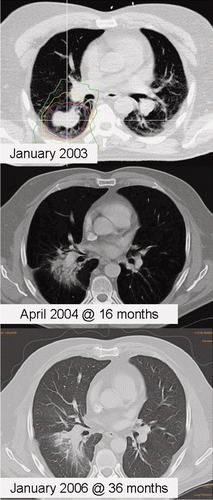
Figure 3. Solitary hilar renal cell cancer metastasis. Dose prescribed was 36 Gy in 12 Gy/fraction. Follow-up CTs document tumor response and slow development of perihilar fibrosis.
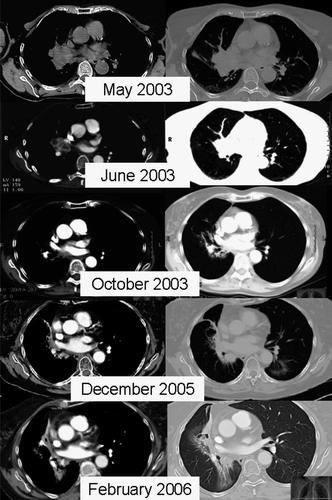
Some degree of wall thickening of major airways without clinical evidence of airflow restrictions was commonly observed. There was one observation of major airway toxicity in a patient treated for a large hilar renal cell carcinoma metastasis. At 36 months, in the absence of clinical symptoms, a routine chest x-ray revealed partial right lower lobe atelectasis. A corresponding bronchoscopy showed narrowing of the right lower lobe bronchus. The patient was offered stent placement; however, as of submission, no interventional procedure had been performed. Please refer to and for a detailed imaging follow-up over time.
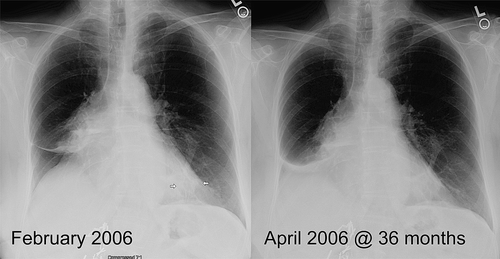
Figure 4. Same patient as in . A chest x-ray at 34 months documents patent airways; however, at 36 months occlusion of the right lower bronchus with subsequent atelectasis of the right lower lobe was observed.
All patients in this cohort received additional treatment for systemic progression of their disease. Additional radiation treatments offered to the patients in this small sample include additional SBRT treatment for new lung lesions (3 patients, one of them twice), whole brain radiation and/or radiosurgery for new brain metastases (4 patients), and treatment for bone metastases (1 patient) and a pancreatic metastasis (1 patient). Only one of three patients alive did not receive additional radiation therapy.
Discussion
In this first report, we focused solely on outcomes of patients treated by SBRT for malignant lung tumors located in close proximity of major airways, and we established that tumor response appears comparable with observations in patients treated for peripheral lung lesions. The local control rate in the present series compares favorably with published data from other series Citation[1–11], Citation[17], Citation[18]. SBRT for lesions within or touching a zone of 2 cm around major airways was associated with a low incidence of pulmonary toxicities. This observation is consistent with reports summarizing early SBRT experience in typically low numbers of patients treated for peripherally located tumors and with limited follow-up.
With respect to major airway toxicity, the Indiana University group reported one case of bronchitis and one case of tracheal necrosis in their series of patients treated for early stage NSCLC in a phase 1 dose escalation trial Citation[3]. Single fraction doses delivered in these two cases were 20 and 24 Gy, respectively, with total doses of 60 and 72 Gy. However, lesion location, proximity to major airways, and time to observation of the toxicities was not reported. These dose levels equate the current dose level assessed in RTOG 0236 and a dose level established to be in excess of the maximally tolerated dose. Song et al. reported two cases of airway occlusion, occurring in a subset of four patients treated for peri-hilar metastases Citation[11]. At 6 and 8 months following SBRT for adrenocortical and ovarian granulosa cell metastases, bronchial occlusion and lobar collapse were observed. Total doses delivered were 35 and 41 Gy, respectively, delivered in 3 fractions over 3 to 6 days. In contrast to the Indiana observations, both fraction doses and total doses in the present series were much lower at 12 and 36 Gy, respectively. However, both dose prescription and dose scheduling were comparable in the Richmond experience. The time to toxicity observation was relatively short in the Richmond series, whereas bronchial occlusion was observed as a late delayed toxicity in the present series. We did not observe any esophageal or vascular toxicity as reported by Wulf et al. Citation[6].
While we propose the efficacy and safety of treating central and hilar lesion by SBRT, the key limitation to the generalization of the findings we report is clearly the limited follow-up. Had we been able to follow our patients longer, the rate of toxicities observed may have increased. One of the major differences between populations treated for stage I NSCLC and patients treated for lung metastases and recurrent disease is the curative versus palliative intent of the treatment. Palliative intent in the present study is understood as the aim to locally control lung disease in patients with this being the sole manifestation of disease or the sole site of disease progression. No patient was actually treated for symptomatic relief. Owing to the nature of systemic disease, the overall survival in the present cohort was limited with the majority of patients ultimately succumbing to systemic disease progression. Most patients had to undergo additional radiation therapy and/or chemotherapy treatments for new disease in follow-up after SBRT. Two patients underwent additional SBRT treatment for new lung disease, which interestingly occurred in the two patients with the longest survival (one patient alive at 38 months, and the other dead at 41.5 months).
Within the limitations of reporting data from a small sample with limited follow-up, we feel that SBRT for centrally located lung lesions contributes to the armamentarium in treating limited systemic disease. At a dose level of 36 Gy delivered in 3 fractions in 48 or 72 hour intervals, treatment of hilar lesions and lesions close to major airways appears feasible, safe and promises local tumor control equivalent to outcomes observed when more peripherally located lung lesions are treated. As major airway toxicities may be observed, the criteria for treating patients under curative intent and with higher doses needs to be more stringent. More data from series of patients already treated and with maturing follow-up will be needed to more clearly establish the probability of normal tissue complications.
References
- Zimmermann FB, Geinitz H, Schill S, et al. Stereotactic hypofractionated radiation therapy for stage I non-small cell lung cancer. Lung Cancer 2005; 48: 107–14
- Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003; 124: 1946–55
- McGarry RC, Papiez L, Williams M, et al. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: Phase I study. Int J Radiat Oncol Biol Phys 2005; 63: 1010–5
- Hof H, Herfarth KK, Munter M, et al. Stereotactic single-dose radiotherapy of stage I non-small-cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 2003; 56: 335–41
- Wulf J, Haedinger U, Oppitz U, et al. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: A noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys 2004; 60: 186–96
- Wulf J, Hadinger U, Oppitz U, et al. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther Onkol 2001; 177: 645–55
- Hiraoka M, Nagata Y. Stereotactic body radiation therapy for early-stage non-small-cell lung cancer: The Japanese experience. Int J Clin Oncol 2004; 9: 352–5
- Nakagawa K, Aoki Y, Tago M, et al. Megavoltage CT-assisted stereotactic radiosurgery for thoracic tumors: Original research in the treatment of thoracic neoplasms. Int J Radiat Oncol Biol Phys 2000; 48: 449–57
- Lee SW, Choi EK, Park HJ, et al. Stereotactic body frame based fractionated radiosurgery on consecutive days for primary or metastatic tumors in the lung. Lung Cancer 2003; 40: 309–15
- Onishi H, Kuriyama K, Komiyama T, et al. Clinical outcomes of stereotactic radiotherapy for stage I non-small cell lung cancer using a novel irradiation technique: Patient self-controlled breath-hold and beam switching using a combination of linear accelerator and CT scanner. Lung Cancer 2004; 45: 45–55
- Song DY, Benedict SH, Cardinale RM, et al. Stereotactic body radiation therapy of lung tumors: Preliminary experience using normal tissue complication probability-based dose limits. Am J Clin Oncol 2005; 28: 591–6
- Nevinny-Stickel M, Sweeney RA, Bale RJ, et al. Reproducibility of patient positioning for fractionated extracranial stereotactic radiotherapy using a double-vacuum technique. Strahlenther Onkol 2004; 180: 117–22
- Fuss M, Salter BJ, Rassiah P, et al. Repositioning accuracy of a commercially available double-vacuum whole body immobilization system for stereotactic body radiation therapy. Technol Cancer Res Treat 2004; 3: 59–67
- Fuss M, Thomas CR, Jr. Stereotactic body radiation therapy: An ablative treatment option for primary and secondary liver tumors. Ann Surg Oncol 2004; 11: 130–8
- Lax I, Blomgren H, Naslund I, et al. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol 1994; 33: 677–83
- Herfarth KK, Debus J, Lohr F, et al. Extracranial stereotactic radiation therapy: Set-up accuracy of patients treated for liver metastases. Int J Radiat Oncol Biol Phys 2000; 46: 329–35
- Blomgren H, Lax I, Naslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995; 34: 861–70
- Blomgren H, Lax I, Goranson H, et al. Radiosurgery for tumors in the body: Clinical experince using a new method. J Radiosurg 1998; 1: 63–74