Abstract
A highly individualized stereotactic body radiotherapy (SBRT) strategy was developed to allow a wide spectrum of patients with liver cancer to be treated. This phase I/II study encompasses individualization of immobilization, radiation planning, PTV margin determination, image guidance strategy and prescription dose. Active breathing control breath hold is used to immobilize the liver when feasible. Image guidance strategies include orthogonal MV images and orthogonal kV fluoroscopy using the diaphragm for a surrogate for the liver, and kV cone beam CT using the liver or tumour for guidance. The prescription dose is individualized to maintain the same estimated risk of radiation-induced liver disease (RILD), based on a normal tissue complication probability (NTCP) model, with a maximum permitted dose of 60 Gy in 6 fractions. Since August 2003, 79 patients with hepatocellular carcinoma (33), intrahepatic cholangiocarcinoma (12) and liver metastases (34) were treated. The median tumour volume was 293 cm3 (2.9–3 088 cm3). The median prescribed dose was 36.6 Gy (24.0 Gy–57.0 Gy) in 6 fractions. The median effective liver volume irradiated was 45% (9–80%). Sixty percent of patients were treated with breath hold to immobilize their liver. Intra-fraction reproducibility (σ) of the liver with repeat breath holds was excellent (1.5 mm); however inter-fraction reproducibility (σ) was worse (3.4 mm). Image guidance reduced the residual systematic and random setup errors significantly.
Hepatic progression from primary and metastatic liver cancer is a substantial cause of morbidity and mortality. Although these cancers can be cured with resection or transplant, surgery is only feasible in the minority of patients. Ablative therapies such as radiofrequency ablation and percutaneous alcohol injections can control tumours less than 5 cm in maximal dimension and away from large vessels, but most tumours are not well suited for these interventions. There is a need for improved therapies for unresectable liver cancers, especially those larger than 5 cm. Advances in tumour imaging, radiation therapy planning and motion management have made it possible for high dose conformal radiation therapy to be used safely in liver cancers Citation[1–3]. Stereotactic body radiation therapy (SBRT) refers to the use of conformal radiation planning to deliver few (generally less than 10) fractions of biologically potent doses of radiation to tumours within the body, outside the brain. When first developed, stereotactic immobilization body frames were a key component of SBRT to aid in patient positioning at the time of treatment. More recently, imaging at the time of radiation delivery, or image guided radiation therapy (IGRT) Citation[4], has become available so that the radiation treatment can be focused directly on the tumour as positioned each day, making the ‘stereotactic’ component of the frames less essential. As the liver can move substantially due to breathing, immobilization of the liver with abdominal compression or breath hold, gating the beam to one phase of the respiratory cycle or tracking the tumour with radiation have all been used during SBRT to reduce the volume of normal tissue required to be irradiated.
The majority of SBRT experience in liver cancers is in patients with small liver tumours (<6 cm) requiring the minority of the liver to be irradiated (e.g. less than 25%). Here, a highly individualized SBRT treatment strategy that allows a wide spectrum of patients with liver cancer will be reported on.
Methods
Patients
Eligible patients for this Institutional Research Ethics Board approved, highly individualized, iso-toxicity SBRT protocol have unresectable primary or metastatic liver cancer, liver enzymes less than six-fold higher than normal, platelet count >80 000 bil/l, Child-Pugh liver score A, >800 cm3 of uninvolved liver, and KPS performance status ≥60. Patients with less than 800 cm3 of non-involved liver, requiring more than 80% of their liver to be irradiated or with prior abdominal radiotherapy precluding the delivery of partial liver irradiation are not eligible.
Study design
The protocol encompasses individualization of immobilization (e.g. breath hold versus free breathing), planning target volume (PTV) margin, radiation planning, image guidance strategy and prescription dose. Such individualization can reduce the volume of uninvolved liver irradiated for an individual patient, so that a higher dose of radiation can be delivered to the tumour, while maintaining the same risk of liver toxicity per patient per risk level.
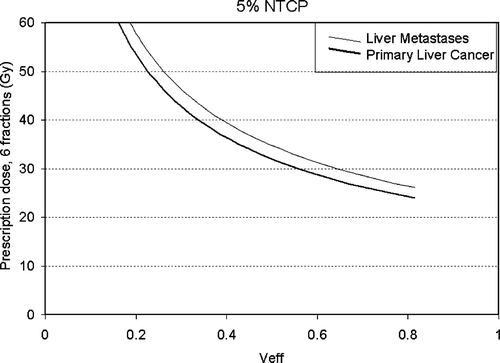
The risk of radiation induced liver disease (RILD) is estimated using a normal tissue complication probability (NTCP) model (described below). The dose escalation strategy is unusual in that the RILD risk level is assigned at enrolment (5%, 10% or 20%), and the prescription dose is determined during the planning process. The number of treatment fractions is fixed at six for all patients, and dose per fraction is escalated.
The primary endpoint of the study is to determine the rate of RILD and other severe toxicity, with stratification based on diagnosis (primary liver cancer versus liver metastases) and effective liver volume (Veff) irradiated (<20%, 20–50%, 50–80%). Three patients have to be treated at each risk level without dose limiting toxicity for each stratum prior to escalation to the next risk level.
Radiotherapy simulation and motion assessment
Simulation consists of an education session, kV fluoroscopy, CT planning and MRI. The education and imaging are usually completed over two days to improve patient comfort and allow repeat measurements of tumour motion during free breathing.
In the education session, a radiation therapist reviews the concepts of organ motion due to breathing and breath hold to reduce motion with the patient. Breath hold using the active breathing control (ABC) device Citation[5] to immobilize the liver to reduce organ motion is investigated, in an effort to reduce the volume of normal tissues that are required to be irradiated. The patient initially practices ABC aided breath holds without imaging to determine their tolerability of repeat 15 s breath holds. On the same day, kV fluoroscopy is used to measure diaphragm motion during free breathing. Stability and reproducibility of the diaphragm relative to the vertebral bodies with repeat ABC breath holds are also measured. A decision is then made regarding whether the patient is to be treated with breath hold or free breathing. The customized immobilization device, with or without ABC breath hold is then designed.
A diagnostic quality tri-phasic CT-scan is acquired in the treatment position using a multi-slice CT-scanner (4 or 16 slices). For patients treated in breath hold using ABC, the planning CT-scan is obtained during ABC aided breath holds (usually end exhale). Non-contrast scans are followed by arterial and/or venous phase image acquisitions at approximately 20 and 60 s post injection respectively (). For free breathing patients, a 4-D CT is obtained, and the images are sorted based on phase of the breathing cycle Citation[6]. Recently, IV contrast-enhanced 4-D CT-scans have been obtained so the liver tumour motion can be assessed over a breathing cycle in addition to the liver motion (). The exhale reconstruction of the 4-D CT is used for radiation planning for these patients, with an expansion to an internal target volume (ITV) based on motion of the tumour during the respiratory cycle, as well as complimentary information about tumour motion from kV fluoroscopy and cine MR.
Figure 1. Exhale breath hold non-contrast and arterial phase IV contrast planning CT-scans of a patient with hepatocellular carcinoma treated during exhale breath hold. The gross tumour volume (GTV) (shown with an arrow) is seen much better on the arterial contrast CT, used for radiation planning.
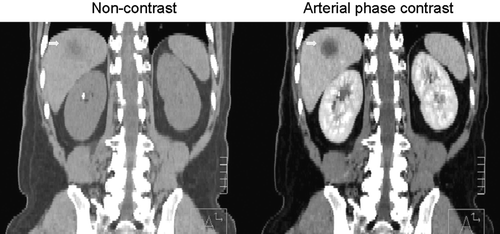
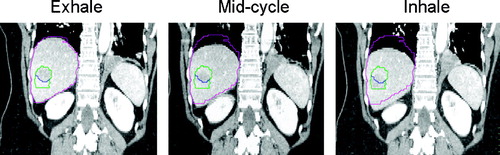
Figure 2. 4-D CT with IV contrast for patient with liver metastases treated during free breathing. Full exhale reconstruction (used for radiation planning), mid cycle and inhale reconstructions displayed. The gross tumour volume (GTV) and liver contour from the exhale reconstruction are shown in blue and pink respectively. The internal target volume (ITV) made from the exhale and inhale phase GTV reconstructions is displayed in green, partially overlapping the exhale phase GTV. An additional 5 mm PTV margin is added to the ITV for setup error.
MR imaging is acquired for tumour motion assessment and for target volume definition. Motion is assessed using cine FIESTA MR sequences orthogonal through the tumour over 30–180 s. Gadolinium contrast MR images are also acquired in voluntary exhale breath-hold, using a T1w fast imaging sequence so that the entire liver can be acquired in a single breath-hold acquired over approximately 20 s.
Once all images are imported to the treatment planning system, the MR images are fused to the CT images using rigid image registration of a limited field of view encompassing the liver. As the liver sometimes changes shape and deforms Citation[7], the merits of deformable image registration in this setting are being investigated.
Treatment planning
The gross target volume (GTV) is defined on exhale breath hold triphasic CT-scans and/or MR imaging acquired in the treatment position. The clinical target volume (CTV) is defined as an 8 mm margin within the liver around the GTV. Two PTVs are defined: one by adding a margin to the GTV to define a PTV to receive the prescribed dose (PTV1), while the second is defined by adding a margin to the CTV, the volume of liver at highest risk for microscopic disease (PTV2). PTV margins are individualized based on use of breath hold or not, reproducibility of breath hold, liver motion due to breathing and reproducibility of motion (minimum permitted PTV margin of 5 mm). All targets and normal structures, including the liver, stomach, small bowel (in the irradiated volume), large bowel (in the irradiated volume), kidneys, heart, and spinal cord, are contoured.
Forward conformal planning is used, with 1 to 2 segments per beam when required to obtain optimal plans. Planning goals are to avoid as much uninvolved liver as possible, and to conform the dose to the target volumes, while respecting normal tissue constraints (). Beam number and placement is individualized to minimize the effective liver volume irradiated, and avoid serial normal tissues such as the stomach and duodenum. An example SBRT plan is shown in .
Figure 3. Radiotherapy plan for a patient with liver metastases treated with 42.6 Gy in 6 fractions. Effective liver volume irradiated was 35%. Planning target volumes (PTVs) around the gross tumour volume (GTV) and clinical target volume (CTV) shown with corresponding isodose lines.
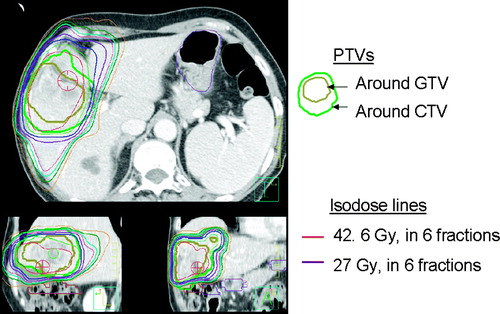
Table I. Protocol dose constraints.
Once an acceptable treatment plan has been obtained, the DVH for the liver minus the GTV is used to estimate the risk of RILD. The liver minus GTV (referred to as liver for the rest of the text) is used, since the GTV is contained within the liver, but is assumed to not contain functional liver tissue. The CTV and PTV are not subtracted as the liver within these volumes is likely functional. Based on the Veff and complication risk, the dose for PTV1 is determined as described below (maximum 60 Gy in 6 fractions). The target dose for PTV2 is 24 Gy in 6 fractions. Inhomogeneity within the PTV is permitted up to 140% of the prescription dose.
Iso-NTCP dose allocation
The risk of RILD was calculated using Lyman- Kutcher-Burman (LKB) NTCP model parameters for RILD obtained from patients treated at 1.5 Gy per fraction (n = 0.97, m = 0.12, TD50 (metastases) = 45.8 Gy, TD50 (primary cancer) = 39.8 Gy) Citation[8]. To correct for the large difference in fractionation between this SBRT protocol and the Michigan hyperfractionated study, the 6 fraction liver dose volume histograms (DVHs) were then normalized to 1.5 Gy per fraction, assuming an αβ/ of 2.5 Gy. The LKB NTCP was then calculated using the Michigan parameters and the normalized liver DVH with a reduction of the DVH to the effective liver volume irradiated (Veff) Citation[9] as follows:where Δvi is a volume bin of a differential DVH, di is the dose to that volume, and dref is the reference dose. The prescription dose is used as the reference dose in this study.
To simplify the process of assigning prescription doses, tables and graphs of Veff and corresponding prescription doses were developed for initial prescription dose determination (). For each plan, the NTCP could not be any higher than the intended risk level (5%, 10% or 20%).
Image guidance
Image guidance strategies used in this protocol include orthogonal MV images and orthogonal kV fluoroscopic images obtained from the treatment unit using the diaphragm for a surrogate for the liver, and kV cone beam CT using the liver or tumour for guidance. Imaging was obtained immediately prior to radiation delivery, in the treatment position (with or without breath hold), with repositioning and verification imaging for offsets greater than 3 mm.
Orthogonal MV image guidance was originally used, using the dome of the diaphragm for guidance in the CC direction, and vertebral bodies for right-left and anterior-posterior positioning. In May 2004, kV cone beam CT became available. Using the cone beam treatment unit, orthogonal kV fluoroscopic projections can also be obtained in the treatment position immediately prior to therapy.
Orthogonal kV fluoroscopic images and kV cone beam CT are acquired in breath hold for patients treated in breath hold and during free breathing for patients treated with free breathing. For the patients treated in ABC breath-hold, volumetric imaging is acquired over one minute. An in-house modification applied to the Elekta Synergy imaging system, termed ‘stop-an-go’, allows the acquisition of a cone beam CT to be acquired in a single 360° rotation that can be paused (typically 3 to 4 times), allowing the patient to have several breaths between sequential breath holds typically 15 to 20 s ().
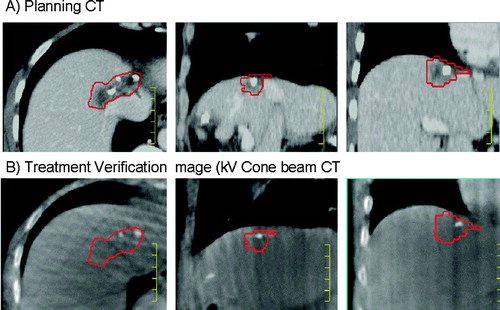
Figure 5. A) Venous phase planning CT for liver metastases radiotherapy planning, and B) verification non contrast kV cone beam CT acquired for image guidance at the time of radiation delivery, registered to the planning CT. Imaging was acquired in exhale breath hold to immobilize the liver. The calcifications in the tumour were used to facilitate alignment of the liver from the cone beam CT to the liver from the planning CT. The gross tumour volume contour on the planning CT is shown on both images.
For the first 13 patients treated with breath hold and imaged with kV orthogonal fluoroscopic projections and kV breath hold cone beam CT images, the kV imaging was acquired in the residual treatment position following MV image guidance. The kV images were reviewed offline and compared to MV images, prior to the routine use of kV image guidance in subsequent patients. For patients with free breathing ranges less than 5 mm, cone beam CT-scans obtained during free breathing are used for image guidance, using liver-to-liver manual matching. For patients not suitable for ABC breath hold treatment, with liver motion more than 5 mm due to breathing, orthogonal kV fluoroscopy is used for image guidance, using the diaphragm for guidance as previously described. Projections from the exhale phase of breathing are used for image matching, as the radiation planning is based on the exhale phase CT with asymmetrical margins for breathing motion. Sorting of the cone beam CT-scans obtained during free breathing (4-D cone beam CT) is being investigated Citation[10].
Results
Patients
From August 2003 to March 2006, 79 patients with hepatocellular carcinoma (HCC) (33), intrahepatic cholangiocarcinoma (12) and liver metastases (34) were treated with highly individualized SBRT. The median age was 64 years (38–92 years). The median tumour volume was 293 cm3 (2.9–3 088 cm3).
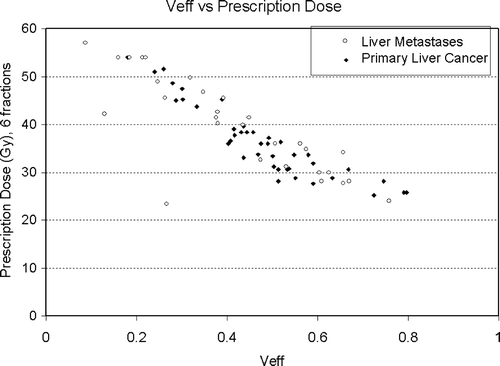
The median effective liver volume irradiated was 45% (9–80%). The median prescribed PTV dose was 36.6 Gy (24.0 Gy–57.0 Gy). A plot of the prescription dose versus the liver effective volume (Veff) is shown in , demonstrating that the full range of effective liver volumes are being investigated.
Figure 6. Prescription dose and effective liver volume of patients with liver metastases (open circle) and primary liver cancer (closed diamond) treated.
The median mean liver dose was 16.8 Gy (1.5–25.2 Gy).
Motion and breath hold
Motion of the liver tumours was largest in cranial caudal (CC) direction, followed by anterior-posterior (AP) and medial-lateral (ML) directions. Analysis of cine MRI from the first 16 patients imaged revealed an average (maximum) tumour motion of 17 mm (29 mm) CC, 9 mm (18 mm) AP, 8 mm (13 mm) ML Citation[11]. The amplitude and pattern of motion due to breathing was variable when assessed using different imaging modalities and when assessed on different days.
Breath hold using ABC for liver immobilization was investigated and considered for treatment in patients with more than 5 mm of liver or tumour motion detected on at least one imaging modality. Sixty percent of patients were screened to be suitable for treatment with breath hold to immobilize their liver during SBRT. Forty percent of patients were not treated with breath hold due to communication concerns, co-morbidities (e.g. lung disease), inability to tolerate repeat breath-holds of 15 s, lack of reproducibility of the diaphragm relative to the vertebral bodies with repeat breath holds or liver motion during free breathing less than 5 mm. In the first 21 patients treated with ABC, the intra-fraction reproducibility (standard deviation, σ) of the liver position relative to the vertebral bodies with repeat breath holds was excellent (1.5 mm). Inter-fraction reproducibility (σ) was worse (3.4 mm), with day-to-day variability in liver position sometimes as large as the free breathing range, providing rationale for daily image guidance Citation[12].
Tumour definition
GTVs as defined using IV contrast CT were compared to GTVs compared using IV contrast MR in 26 patients Citation[13]. Median percentage concordance volumes were 81% (77–86%) in metastases, 77% (60–88%) in hepatocellular carcinoma and 64% (25–85%) in cholangiocarcinoma. The median percentage of CT and MR tumour surface area that differed by ≥5 mm was 26% (1–86%). This study also revealed that liver deformation can occur despite attempts to control for liver position with exhale breath hold and accounting for deformation reduces the differences between GTVs defined on CT and MR.
Image guidance
For patients treated with breath hold, image guidance using MV orthogonal imaging, with the diaphragm as a surrogate for the liver tumour, reduced the residual systematic and random setup errors significantly. Analysis of 405 images from 20 patients revealed that repositioning was needed in 109 of 120 treatment fractions to correct for offsets in position greater than 3 mm. Image guidance and repositioning reduced the population random setup errors (σ) from 6.5 mm (CC), 4.7 mm (AP), and 4.2 mm (ML) to 2.5 mm, 2.9 mm and 2.8 mm respectively. Image guidance also reduced the standard deviation of the distribution of systematic deviations (Σ) from 5.1 mm (CC), 3.4 mm (ML) and 3.1 mm (AP) to 1.4 mm, 2.0 mm and 1.9 mm respectively Citation[14].
Thirteen patients treated with breath hold were imaged with orthogonal MV imaging, kV fluoroscopy and kV breath hold cone beam CT in their residual treatment position. Analysis of the cone beam CT obtained in the treatment position following MV image guidance, revealed that the liver was within 5 mm of its planned position 67% of the time, with occassional offsets as high as 10 mm Citation[15]. In subsequent patients, orthogonal kV fluoroscopic imaging and/or kV cone beam CT were used for guidance instead of MV image guidance to improve accuracy with reduced dose.
Outcomes
The primary end point of this study is to determine the rate of radiation-induced liver toxicity and other related severe toxicity occurring within three months of treatment completion. Secondary endpoints are to determine a better understanding of the partial volume tolerance of the liver to six fraction SBRT and measure late toxicity, local control, hepatic control, progression free survival, survival and quality of life in these patients.
As of June 2006, dose-limiting toxicity has not been observed, and individualized, image guided, iso-NTCP liver SBRT appears feasible. Accrual to this study continues, and clinical outcomes will be presented in detail elsewhere.
Discussion
The concept for use of NTCP models to individualize therapy and facilitate dose allocation was first introduced by Ten Haken et al. at the University of Michigan Citation[16], demonstrating this as a feasible and safe method of allocating dose for liver cancers Citation[17]. Analysis of over 200 patients with liver cancer treated with conformal hyperfractionated radiation therapy at the University of Michigan has led to a better understanding of the partial volume tolerance of the liver to radiation therapy Citation[8]. Outcomes following conformal radiation therapy for both primary and metastatic unresectable focal liver cancers are encouraging Citation[1], Citation[3], Citation[18], Citation[19].
Advantages of SBRT for liver cancer include the potential radiobiological therapeutic advantages of a short radiation treatment time and high dose per fraction, as well as increased patient convenience, and more efficient resource utilization. SBRT for liver cancers was first reported on by Blomgren et al. the early 1990s Citation[20]. RILD was observed infrequently following SBRT, increasing the awareness that SBRT needs to be used cautiously. Since then, others have used SBRT for liver cancers safely in a variety of fractionations Citation[21–24], predominant for tumours less than 6 cm requiring a small effective volume of liver to be irradiated. This clinical experience is consistent with the fact that the liver is a parallel functioning organ, and that very high doses of radiation can be delivered to the liver with little toxicity, as long as enough volume of liver can be spared. Despite this clinical experience, the partial volume tolerance of the liver to hypofractionated radiation therapy, or SBRT, is not well established. Given that the majority of liver tumours unsuitable for other therapies require substantial volumes (i.e. more than 30%) of the liver to be irradiated, we developed an iso-toxic protocol, using a 6 fraction SBRT schedule to allow a wide range of liver tumour sizes and irradiated liver volumes.
This iso-toxicity study should help establish the partial volume tolerance of the liver to hypofractionated radiation therapy. As the six-fraction SBRT schedule is very different from the fractionation used in which the liver NTCP model was developed, a correction for the change in dose per fraction was made. It is possible (perhaps likely) that this study will not confirm the validity or accuracy of the LKB NTCP model, or determine the liver α/β ratio. However, if toxicity rates are confirmed to be low at the completion of this study, this dose allocation method will be useful for safely guiding dose allocation, even if the risk of toxicity is overestimated.
Technical planning factors are also individualized in this protocol (e.g. planning, immobilization, image guidance etc), which allow a reduction in the required PTV margin, and may facilitate dose escalation. Escalated doses of radiation have been associated with improved local control and survival in prior radiation therapy series Citation[1], Citation[19]. Although, the required SBRT doses for optimal primary and metastatic liver tumour control are not yet clearly established, this study should help in the understanding of dose response once mature outcome data is available. As introduction of technological advances to the clinic occur, they can be incorporated into this study, with modification of individual prescription doses based on volume of liver irradiated.
There are many advantages of image guidance for liver SBRT. The immediate benefit for an individual patient is that geometric uncertainty is reduced, allowing for smaller irradiated volumes and greater accuracy in the delivery of the intended dose. This can reduce the risk of toxicity and/or facilitate delivery of higher doses to the tumour. Furthermore, since variability due to geometric uncertainty is significantly reduced, the impact of dose and other prognostic factors on toxicity risk and local control should be able to be better established.
In conclusion, an iso-toxic SBRT protocol has been developed that allows a wide spectrum of patients to be irradiated. Future work intends to evaluate the available toxicity and efficacy data in detail, to determine the α/β for liver, to evaluate the safety of different fractionation schemes, and to better understand the partial volume tolerance of liver to SBRT. Efficiency of the SBRT process and patient comfort will also continue to be improved. Once safety and optimal doses of SBRT has been established, combined modality treatment with chemotherapy and/or novel biologic therapies can be pursued in addition to randomized trials of SBRT for liver cancers.
This study was supported in part from grants provided by Elekta Oncology Systems. Dr. Dawson is a recipient of an ASCO career development award.
References
- Ben-Josef E, Normolle D, Pan C, Tatro D, Ten Haken RK, Knol J, et al. A Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol 2005; 23: 8739–47
- Dawson LA, Lawrence TS. The role of radiotherapy in the treatment of liver metastases. Cancer J 2004; 10: 139–44
- Hawkins M, Dawson L. From palliation to cure: Radiotherapy for hepatocellular carcinoma. Cancer 2006; 106: 1653–63
- Dawson L, Sharpe M. Image guided radiation therapy: Rationale, benefits and cautions. Lancet Oncol 2006; (in press).
- Wong JW, Sharpe MB, Jaffray DA, Kini VR, Robertson JM, Stromberg JS. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys J1. Int J Radiat Oncol Biol Phys J2-Red J 1999; 44: 911–9
- Rietzel E, Pan T, Chen G. Four-dimensional computed tomography: Image formation and clinical protocol. Med Phys 2005; 32: 874–89
- Brock KK, Dawson LA, Sharpe MB, Moseley DJ, Jaffray DA. Feasibility of a novel deformable image registration technique to facilitate classification, targeting, and monitoring of tumor and normal tissue. Int J Radiat Oncol Biol Phys 2006; 64: 1245–54
- Dawson LA, Normolle D, Balter J, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation induced liver disease using the lyman NTCP model. Int J Radiat Oncol Biol Phys 2002; 53: 810–21
- Kutcher GJ, Burman C, Brewster L, Goitein M, Mohan R. Histogram reduction method for calculating complication probabilities for three-dimensional treatment planning evaluations. Int J Radiat Oncol Biol Phys 1991; 21: 137–46
- Sonke JJ, Zijp L, Remeijer P, van Herk M. Respiratory correlated cone beam CT. Med Phys 2005; 32: 1176–86
- Dawson L, Eccles C, Kirilova A, Brock K. Three dimensional motion of liver tumours using cine MRI compared to liver motion assessed at fluoroscopy. A483. Rad. Oncol 2004; 73(Suppl 1)S214
- Eccles CL, Brock KK, Hawkins M, Bissonnette JP, Dawson LA. Reproducibility of liver position using active breathing coordinator for liver cancer radiotherapy. Int J Radiat Oncol Biol Phys 2006; 64(3)751–759
- Voroney J, Brock K, Eccles C, Haider M, Dawson LA. Prospective comparison of CT and MR for liver cancer delineation using deformable image registration. Int J Radiat Oncol Biol Phys 2006; (in press).
- Dawson LA, Eccles C, Bissonnette JP, Brock KK. Accuracy of daily image guidance for hypofractionated liver radiotherapy with active breathing control. Int J Radiat Oncol Biol Phys 2005; 62: 1247–52
- Hawkins M, Brock K, Eccles C, Moseley D, Jaffray DA, Dawson LA. Assessment of residual error in liver position using kV cone-beam CT for liver cancer high precision radiation therapy. Int J Radiat Oncol Biol Phys 2006; (in press).
- Ten Haken RK, Martel MK, Kessler ML, Hazuka MB, Lawrence TS, Robertson JM, Turrisi AT, Lichter AS. Use of Veff and iso-NTCP in the implementation of dose escalation protocols. Int J Radiat Oncol Biol Phys 1993; 27: 689–95
- McGinn CJ, Ten Haken RK, Ensminger WD, Walker S, Wang S, Lawrence TS. Treatment of intrahepatic cancers with radiation doses based on a normal tissue complication probability model. J Clin Oncol 1998; 16: 2246–52
- Mohiuddin MCE, Ahmad N. Combined liver radiation and chemotherapy for palliation of hepatic metastases from colorectal cancer. J Clin Oncol 1996; 14: 722–8
- Seong JPH, Han KH, Chon CY. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: A retrospective study of 158 patients. Int J Radiat Oncol Biol Phys 2003; 55: 329–36
- Blomgren H, Lax I, Naslund I, Svanstrom R. Steretactic high dose fraction radiation therapy of extracranial tumours using an acclerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995; 34: 861–70
- Fuss M, Thomas CR. Stereotactic body radiation therapy: An ablative treatment option for primary and secondary liver tumors; Ann Surg Oncol 11(2); 2004. p. 130–8.
- Herfarth KK, Debus J, Wannenmacher M. Stereotactic radiation therapy of liver metastases: Update of the initial phase-I/II trial. Front Radiat Ther Oncol 2004; 38: 100–5
- Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 2005; 62: 1371–8
- Wulf J, Hadinger U, Oppitz U, Thiele W, Flentje M. Stereotactic boost irradiation for targets in the abdomen and pelvis. Radiol Oncol 2004; 70(1)31–6